��Ŀ����
��14�֣���ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ�� 2CH3OH+3O2+4KOH 2K2CO3+6H2O ��д���пհף�
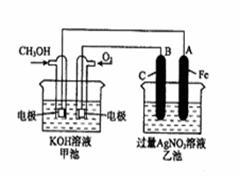
��1����д���ס������ص����ơ������ ���ҳ��� ��
��2���׳���ͨ��CH3OH�ĵ缫������ ���缫��Ӧ����ʽΪ ���ҳ���B��ʯī���缫�������� ��
��3���������У��ҳ���ҺpH�ı仯Ϊ�������ߡ��������͡����䡱 �� ��
��4�����ҳ���A��Fe��������������5.40gʱ���׳�������������O2 mL����״���£�

��1����д���ס������ص����ơ������ ���ҳ��� ��
��2���׳���ͨ��CH3OH�ĵ缫������ ���缫��Ӧ����ʽΪ ���ҳ���B��ʯī���缫�������� ��
��3���������У��ҳ���ҺpH�ı仯Ϊ�������ߡ��������͡����䡱 �� ��
��4�����ҳ���A��Fe��������������5.40gʱ���׳�������������O2 mL����״���£�
��14�֣�
��1��ԭ��ء�����
��2��������CH3OH �� 6 e- + 8OH�� = CO32�� + 6H2O ����
��3������
��4��280
��1��ԭ��ء�����
��2��������CH3OH �� 6 e- + 8OH�� = CO32�� + 6H2O ����
��3������
��4��280
��

��ϰ��ϵ�д�
�����Ŀ
 Fe2+ + H2��
Fe2+ + H2��






 4e����4OH��
4e����4OH��