��Ŀ����
��ͼ��ij��ѧʵ��С����̵���ص������Լ���ǩ�IJ������ݡ���С���ڿ��������л�����¡���Ϣ��Ũ���ḯʴ�Ժ�ǿ������ˮʱ�ͷŴ������ȡ�Ũ�����е�����ֲ����룺���� ��ѧ��(CP) (500mL) Ʒ�������� ��ѧʽ��H2SO4 ���ԭ��������98 �ܶȣ�1.84g/cm3 ����������98% |
H2SO4![]() H++
H++![]()
![]()
![]() H++
H++![]()
���ڸ��Լ�ƿ���ܷ�ǩ���������ǻ��ɸ����������������ǩ������������չ�о���
����Ϊ������һ�־��ܵ�������������Һ��c(H+)����c(H+)=36.8 mol/L�������Һ����������Ϊ0.98��
����Ϊ����ʹ�С����ܵ�������������Ҳ�����У������á����������������ⶨ���������룺ȡһ����������������BaCl2��Ӧ�����ˡ�ϴ�ӡ�����������Ƴ�����������
�������á��к͵ζ������в������������£�A.ȷ��ȡһ����������ᣬ����������ˮϡ�ͣ�B.��ϡ�ͺ���Һ�е���2��3�η�̪��C.��һ��Ũ�ȵ�NaOH��Һ�ζ���ֱ�����ֵζ��յ�Ϊֹ��D.��¼���ĵ�NaOH��Һ��������ش��������⣺
(1)���ƶϡ���ͬѧ�ķ��������С���������________________________________________��
(2)�ҷ����Ĺؼ����������㣺��ȷ�������������ȫ��Ӧ����ϴ�ӳ�����ȷ����������������ʡ���ʵ��������϶������������ȫ��Ӧ?��___________________________________������ڹ�������ϴ�ӳ���?��____________________________________________________��
(3)���ϡ��Ũ������Һ? _________________________________________________________��
(4)����ʵ���У��ֱ�ȡ����5.00mL����5.0mol/L NaOH�ζ����Σ��յ�ʱ�õ���NaOH���������Ϊ35.65 mL��40.62mL��35.55 mL����ͨ������˵���������Ƿ�ϸ�
(1)Ũ��������������ʣ�������ȫ����
(2)���ϲ���Һ��(��ȡ�����ϲ���Һ����С�Թ���)���μ�BaCl2��������ٲ���������˵�������Ѿ���ȫ����©����ע����������ˮ��ʹˮ��û���������ˮ������ظ���������
(3)ȡһ�������ˮ�����ձ��У���Ũ���Ỻ������ˮ�У��ߵ��߽���
(4)H2SO4��������2NaOH
1 2
c��5��10-3 L 5.0moL/L��(35.65+35.55)��10-3 L/2
c=17.8 mol/L��18.4 mol/L
��ˣ���Ũ������ϱ�ǩҪ��

 ��������һ���þ�ϵ�д�
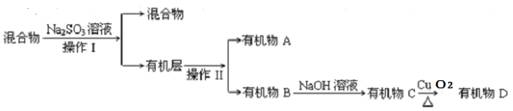
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д� C2H6+C2H4�� C4H10
C2H6+C2H4�� C4H10 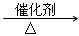 CH4+C3H6��
CH4+C3H6��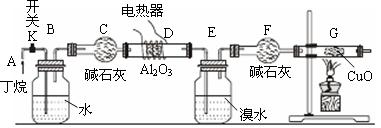
 ˮ����)���ٰ���������ʵ�飺
ˮ����)���ٰ���������ʵ�飺 �ٲ���I������II�ֱ��� �� �� ��
�ٲ���I������II�ֱ��� �� �� �� 
 ������ ��
������ ��  C2H6+C2H4�� C4H10
C2H6+C2H4�� C4H10  CH4+C3H6��
CH4+C3H6��