��Ŀ����
��11�֣�������һ���������ѽ�ɰ����ַ�ʽ���У�
C4H10 C2H6+C2H4�� C4H10
C2H6+C2H4�� C4H10  CH4+C3H6��
CH4+C3H6��
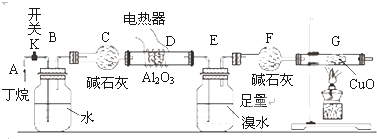
��ͼ��ij��ѧ��ȤС����ж����ѽ��ʵ�����̡�(ע��CuO�ܽ���������CO2��H2O��G����װ��������أ�ʡ��)

����ͼ����װ�ú�����е�ʵ������У� �ٸ�D�� Gװ�ü��ȣ� �ڼ������װ�õ������ԣ� ���ų�װ���еĿ����ȡ�����
���������������Ⱥ�˳��������____ _____��
����������������___ _ ��
д������������ͭ��Ӧ�Ļ�ѧ����ʽ ��
��Bװ�������������____________ ______��
������Eװ���еĻ����(��ˮ����)�� �ٰ���������ʵ�飺
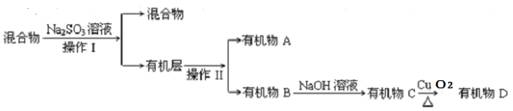
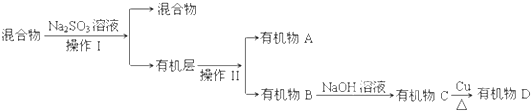
�ٲ���I������II�ֱ��� �� ��
����֪D����˴Ź�����ͼ��ֻ��һ�ַ壬��D�Ľṹ��ʽ ��
��Na2SO3��Һ�������� ��
(5)�ٶ�������ȫ�ѽ⣬��(E+F)װ�õ��������ȷ�Ӧǰ������0.7 g�� Gװ�õ�����������1.76 g��������ѽ�����м������������ʵ���֮��n (CH4) : n (C2H6) =______(�ٶ�����D��Gװ���е���������ȫ��Ӧ)
(11��)��1���ڡ� �ۡ� �� (1��)
��2������(1��)��CH4+4CuO��CO2+2H2O+4Cu(2��)
��3���۲졢���ƶ������������(1��)
��4���� ��Һ������(��1��,��2��) �� OHC-CHO (1��) �� ��ȥ�л������ܽ��Br2(1��)
 ��5��1:1(2��)
��5��1:1(2��)
��������

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�


 C2H6+C2H4�� C4H10
C2H6+C2H4�� C4H10  CH4+C3H6��
CH4+C3H6��

 C2H6+C2H4�� C4H10
C2H6+C2H4�� C4H10
 CH4+C3H6��
CH4+C3H6��
 �ٲ���I������II�ֱ��� ��
��
��
�ٲ���I������II�ֱ��� ��
��
��