��Ŀ����
����Ŀ����ͼ��ʾϸ����ijЩ�л����Ԫ����ɺ��ܹ�ϵ������A��B����Ԫ�أ������������ӣ�ͼ��X��Y��Z��P�ֱ�Ϊ�����������ӵĻ�����λ����ش��������⣺
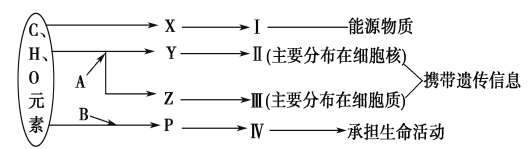
(1)ͼ��X��________��I��С����������Ҫ��ָ______________��
(2)ͼ��Z��________��ʹ�ü��̡�������(����)���ȾҺȾɫ����ʹ�����________ɫ��
(3)ͼ��P�ĽṹͨʽΪ________��д����P�γɢ��Ľṹ���______________��
(4)��͢����߶��ж����ԣ����߶����ԵĹ�ϵ��ǰ��________���ߡ�
(5)����ϸ���Ļ������У����������������Լ��ٵ���Ҫ��________��
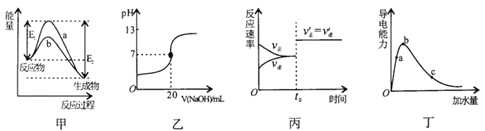
���𰸡������� ���ۺ��Ǻ������ ��������������������ʾ���ˮ
��������������������ʾ���ˮ
��������
���������ͼʾ������֪��A��BΪ��Ԫ�ء�XΪ�����ǡ�YΪ���������ᡢZΪ���Ǻ����ᡢPΪ���������ʾ���ࡢ����ʾDNA������ʾRNA������ʾ��������
��1������ͼ֪��X����C��H��O��ɵ�С�������ʣ��ұ���Ϊ��������ȼ������X�������ǣ��������������γɵĶ��ǣ���С������ϸ������Ҫ�ǵ�����
��2�����Ĺ�����Я���Ŵ���Ϣ������Ϊ���ᣬZ��Ҫ�ֲ���ϸ�����У������ں��Ǻ����ᣬ����������ɽ�III��RNA��Ⱦ�ɺ�ɫ��
��3��ͼ��PΪ�����ʵĻ�����ɵ�λ����������ͨʽΪ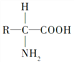 ����P�γ����Ľṹ���Ϊ����������������������
����P�γ����Ľṹ���Ϊ����������������������
��4������mRNA����ͨ�������γɵ����ʣ����III��RNA���Ķ����Ծ����˵����ʵĶ����ԡ�
��5������ϸ���Ļ������У���������������˥�ϵ�ϸ�������Լ��ٵ���Ҫ��ˮ��

����Ŀ����ѧ��Ӧԭ���ڿ��к��������й㷺Ӧ�á�
��1�����������Ƚ���(��Ҫ�ɷ�ΪCO��CH4��H2)��SO2��һ�������»�ԭΪ�������������������֪��
��C(s)+O2(g)=CO2(g) ��H1=-393.5 kJ��mol-1
��CO2(g)+C(s)=2CO(g) ��H2= + 172.5 kJ��mol-1
��S(s)+O2(g)=SO2(g) ��H3=- 296.0 kJ��mol-1
CO��SO2��ԭΪ��������Ȼ�ѧ����ʽΪ________________________��
��2��CO�����ںϳɼ״���һ���¶��£������Ϊ2 L���ܱ������м���CO��H2��������ӦCO(g)+2H2(g)![]() CH3OH(g)��5���Ӻ�ﵽƽ���ø�������ʵ������£�
CH3OH(g)��5���Ӻ�ﵽƽ���ø�������ʵ������£�
���� | CO | H2 | CH3OH |
���ʵ�����mol�� | 1.8 | 2.0 | 1.2 |
�ٷ�Ӧ�ﵽƽ��ʱ��CO��ת����Ϊ_______��5��������H2��ʾ������Ϊ________
�ڸ÷�Ӧ��ƽ�ⳣ��K=__________��
�ۺ��º��������£�����˵����Ӧ�Ѵﵽƽ��״̬����__________(����)��
A��v��(CO)=2v��(H2) B�����������ܶȲ���
C����������ƽ����Է����������� D��CO��H2��Ũ��֮��Ϊ1:2
E.��λʱ���ڣ�ÿ����1mol H2����2mol CH3OH
�������������ѹ����1L����ﵽ��ƽ��ʱc(H2)��ȡֵ��Χ��_____________��
������������������䣬�ٳ���0.6 mol CO ��0.4 mol CH3OH����ʱv�� __________v��(� >�� < ����= ��)��