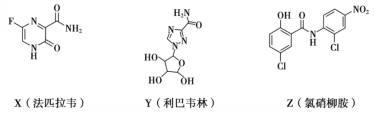
��Ŀ����
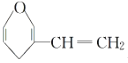
����Ŀ����1��������ϩ�����PVC�����ֲ����������ʳƷ��װ����������İ�ȫ����һ����Ӱ�죬д�����ɾ�����ϩ�Ļ�ѧ����ʽ��____��
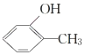
��2�����ȡ������![]() �����Ա�����KMnO4��Һ��������
�����Ա�����KMnO4��Һ��������![]() ���������R��ֱ���뱽�����ӵ�̼ԭ��û��C��H���������ױ������õ�
���������R��ֱ���뱽�����ӵ�̼ԭ��û��C��H���������ױ������õ�![]() �����з���ʽ��C11H16�����һȡ�������������Ա�������Ϊ
�����з���ʽ��C11H16�����һȡ�������������Ա�������Ϊ![]() ��ͬ���칹�干��__�֣���д��һ�ֲ��ܱ�������Ϊ
��ͬ���칹�干��__�֣���д��һ�ֲ��ܱ�������Ϊ![]() ��ͬ���칹��Ľṹ��ʽ___��
��ͬ���칹��Ľṹ��ʽ___��
��3����֪ ����д������ϩ��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ����������Ľṹ��ʽ��___��
����д������ϩ��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ����������Ľṹ��ʽ��___��
��4�����и��������У������Ժ��ֱ�����ϣ�ȡnmolʹ֮���ȼ�գ�������������CO2������Ϊ��ֵ����____��
A.C2H4��C2H4O B.C2H6��C3H6O2 C.C2H6��C3H6O D.C2H4��C2H6O
���𰸡�![]() 7
7 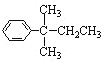
![]() D
D
��������
��1������ϩ��̼̼˫�����ɷ����Ӿ۷�Ӧ��
��2������ʽ��C11H16�����һȡ�������������Ա�������Ϊ![]() ����-C5H11����Ŀ������ͬ���칹�����Ŀ��������R��ֱ���뱽�����ӵ�̼ԭ��û��C-H���������ױ������õ�
����-C5H11����Ŀ������ͬ���칹�����Ŀ��������R��ֱ���뱽�����ӵ�̼ԭ��û��C-H���������ױ������õ�![]() �����
�����
��3�����������Ϣ�ж�������Ϊ![]() ��
��
��4�����������֪���л��������Ժ������ʵ����ı�����ϣ�ֻҪ�����ʵ���һ������ȫȼ��������������������CO2����Ϊһ�㶨ֵ���ɼ����л�������ʵ�����Ϊ1mol������ȫȼ�����ĵ����������ʵ���������CO2��������ȣ������Ժ������ʵ����ı�����ϣ���ȫȼ��������������������CO2����Ϊһ�㶨ֵ��������������������Ϊx+ ![]() ��xΪCԭ������yΪHԭ���������㣬���л����к���Oԭ�ӣ����÷���ʽ��д�ķ������㣬CO2�ɸ���Cԭ�������㡣
��xΪCԭ������yΪHԭ���������㣬���л����к���Oԭ�ӣ����÷���ʽ��д�ķ������㣬CO2�ɸ���Cԭ�������㡣
��1������������ϩ�Ļ�ѧ����ʽΪ![]() ��
��
��Ϊ![]() ��
��
��2������ʽ��C11H16�����һȡ�������������Ա�������Ϊ![]() ����-C5H11����Ŀ������ͬ���칹�����Ŀ��
����-C5H11����Ŀ������ͬ���칹�����Ŀ��
���������-C5H11��-CH2CH2CH2CH2CH3��-CH(CH3)CH2CH2CH3��-CH(CH2CH3)2��-CH2CH��CH3��CH2CH3��-CH2CH2CH(CH3)2��-CH(CH3)CH(CH3)2��-CH2C(CH3)3����7�֣��������R��ֱ���뱽�����ӵ�̼ԭ��û��C-H���������ױ������õ�![]() ���ýṹΪ
���ýṹΪ ��
��
����7�� ��
��
��3������ϩ�к���̼̼˫������![]() ��֪����Ӧ�ɱ�ʾΪ
��֪����Ӧ�ɱ�ʾΪ![]() ��������ṹ��ʽΪ
��������ṹ��ʽΪ![]() ��
��
��Ϊ![]() ��
��
��4��
A��1molC2H4��ȫȼ������3mol����������2molCO2��1molC2H4O��ȫȼ������
B��1molC2H6��ȫȼ������3.5mol����������2molCO2��1molC3H6O2��ȫȼ������3.5mol����������3molCO2����������ȣ�����CO2��������ȣ���B���������⣻
C��1molC2H6��ȫȼ������3.5mol����������2molCO2��1molC3H6O��ȫȼ������4mol����������3molCO2��������������CO2����������ȣ���C���������⣻
D��1molC2H4��ȫȼ������3mol����������2molCO2��1molC2H6O��ȫȼ������3mol����������2molCO2��������������CO2�������߾���ȣ����������Ժ��ֱ�����ϣ�ȡnmolʹ֮���ȼ�գ�������������CO2����Ϊ��ֵ����D�������⣻
��ΪD��

 �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д� ���ɿ��õ�Ԫ����AB��ϵ�д�
���ɿ��õ�Ԫ����AB��ϵ�д�