��Ŀ����
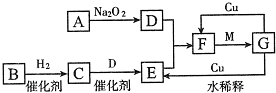
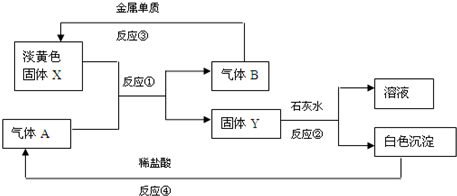
�����ķǽ�����ɫ���嵥��A�볣����������B���ڼ��������·�Ӧ���ɻ�����C��C��ˮ��Ӧ���ɰ�ɫ����D������E��D��������ǿ�ᣬҲ������ǿ�E������������ȼ�ղ����̼�������G��G�ڴ������ܵ���������γɣ�E����������������Һ���յõ���ɫ��ҺF����ҺF�ڿ����г��ڷ���������������Ӧ����Һ��ǿ���ԣ�������֮һΪH��H�����Ԫ����F��ͬ����ṹ�ͻ�ѧ����������������ƣ���Һ�Ի�ɫ����ش��������⣺
��1��B������������Һ��Ӧ�Ļ�ѧ����ʽΪ��______��
��2��G�������������������·�Ӧ����������ɱ�����������ȣ��÷�Ӧ����������Ϊ______��������2mol��������ʱ��ת�Ƶ���______mol��
��3����ҺF�ڿ����г��ڷ�������H�Ļ�ѧ��Ӧ����ʽΪ��______��
��4��H����Һ��ϡ���ᷴӦ����������Ϊ______��
��1��B������������Һ��Ӧ�Ļ�ѧ����ʽΪ��______��
��2��G�������������������·�Ӧ����������ɱ�����������ȣ��÷�Ӧ����������Ϊ______��������2mol��������ʱ��ת�Ƶ���______mol��
��3����ҺF�ڿ����г��ڷ�������H�Ļ�ѧ��Ӧ����ʽΪ��______��
��4��H����Һ��ϡ���ᷴӦ����������Ϊ______��
�����ķǽ�����ɫ���嵥��ΪS����AΪS��D��������ǿ�ᣬҲ������ǿ���DΪAl��OH��3��˵��BΪAl��������CΪAl2S3����ˮ��Ӧ����Al��OH��3��H2S����EΪH2S��������������ȼ�ղ����̼�������SO2����GΪSO2��H2S����������������Һ���յõ���ɫ��ҺNa2S����FΪNa2S��H�����Ԫ����Na2S��ͬ����ṹ�ͻ�ѧ����������������ƣ�ӦΪNa2S2����
��1��BΪAl������NaOH��Һ��ӦNaAlO2��H2����Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2��GΪSO2�����л�ԭ�ԣ������ǿ�����Ե������Ʒ�Ӧ���ɶ������ȣ���SO2������Ϊ���ȶ������ʣ�ӦΪ�����ƣ�Na2SO4������������ClԪ�صĻ��ϼ�Ϊ+5�ۣ�����������ClԪ�صĻ��ϼ�Ϊ+4�ۣ�������2mol��������ʱ��ת�Ƶ���Ϊ2mol����5-4��=2mol��
�ʴ�Ϊ�������ƣ�Na2SO4����2��
��3����ҺNa2S�ڿ����г��ڷ���������������Ӧ����Һ��ǿ���ԣ�˵����NaOH���ɣ�������֮һΪNa2S2������ܷ������·�Ӧ��2Na2S+O2+2H2O=4NaOH+2S��Na2S+S=Na2S2���ܷ�ӦΪ
4Na2S+O2+2H2O=4NaOH+2Na2S2��
�ʴ�Ϊ��4Na2S+O2+2H2O=4NaOH+2Na2S2����2Na2S+O2+2H2O=4NaOH+2S��Na2S+S=Na2S2����
��4��Na2S2���ȶ�����������������������������ԭ��Ӧ����S��H2S���ɹ۲쵽dz��ɫ�����ͣ���������ζ�ģ����壬��Һ��ɫ�ɻ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ����Һ�ɻ�ɫ��Ϊ��ɫ������dz��ɫ�����ͣ���������ζ�ģ����壮
��1��BΪAl������NaOH��Һ��ӦNaAlO2��H2����Ӧ�Ļ�ѧ����ʽΪ2Al+2NaOH+2H2O=2NaAlO2+3H2����
�ʴ�Ϊ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2��GΪSO2�����л�ԭ�ԣ������ǿ�����Ե������Ʒ�Ӧ���ɶ������ȣ���SO2������Ϊ���ȶ������ʣ�ӦΪ�����ƣ�Na2SO4������������ClԪ�صĻ��ϼ�Ϊ+5�ۣ�����������ClԪ�صĻ��ϼ�Ϊ+4�ۣ�������2mol��������ʱ��ת�Ƶ���Ϊ2mol����5-4��=2mol��
�ʴ�Ϊ�������ƣ�Na2SO4����2��
��3����ҺNa2S�ڿ����г��ڷ���������������Ӧ����Һ��ǿ���ԣ�˵����NaOH���ɣ�������֮һΪNa2S2������ܷ������·�Ӧ��2Na2S+O2+2H2O=4NaOH+2S��Na2S+S=Na2S2���ܷ�ӦΪ
4Na2S+O2+2H2O=4NaOH+2Na2S2��
�ʴ�Ϊ��4Na2S+O2+2H2O=4NaOH+2Na2S2����2Na2S+O2+2H2O=4NaOH+2S��Na2S+S=Na2S2����
��4��Na2S2���ȶ�����������������������������ԭ��Ӧ����S��H2S���ɹ۲쵽dz��ɫ�����ͣ���������ζ�ģ����壬��Һ��ɫ�ɻ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ����Һ�ɻ�ɫ��Ϊ��ɫ������dz��ɫ�����ͣ���������ζ�ģ����壮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


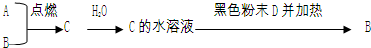

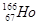 ����Ч�����Ƹΰ�����ͬλ��ԭ�Ӻ��ڵ�������
����Ч�����Ƹΰ�����ͬλ��ԭ�Ӻ��ڵ�������