��Ŀ����
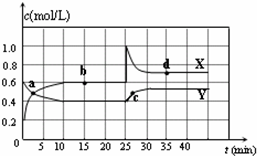
 ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ��
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ�����Ϊ2 L�ĺ����ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ����1��ͼ�й�����������X��Y�����б�ʾNO2Ũ����ʱ��仯��������
X
X
��a��b��c��d�ĸ����У���ʾ��ѧ��Ӧ����ƽ��״̬�ĵ���b��d
b��d
����2����ǰ10min����NO2��ʾ�Ļ�ѧ��Ӧ����v��NO2��=
0.04
0.04
mol?L-1?min-1���ڷ�Ӧ2NO2��g��?N2O4��g����b���ƽ�ⳣ��K��b��=
| 10 |
| 9 |
| 10 |
| 9 |
�۷�Ӧ2NO2��g��?N2O4��g����d���ƽ�ⳣ��K��d����b���ƽ�ⳣ��K��b���Ĺ�ϵ��K��d��
=
=
K��b�������������=������������3���پ�ͼ��������25minʱ��ȡ�Ĵ�ʩ��
C
C
������ţ���A��������� B����С�������
C������һ������NO2 D������һ������N2O4
������35minʱ�������¶Ȳ��䣬������С�����������1L���������ɫ�仯������
�ȱ�����dz������ԭƽ����ɫ��
�ȱ�����dz������ԭƽ����ɫ��
����������1�����ݷ�Ӧ����ʽ2NO2��g�� N2O4��g����֪��NO2��Ũ�ȱ仯��N2O4Ũ�ȱ仯����2�����ݴ��жϣ�
N2O4��g����֪��NO2��Ũ�ȱ仯��N2O4Ũ�ȱ仯����2�����ݴ��жϣ�
���ʵ�Ũ�Ȳ������仯ʱ��ʾ��ѧ��Ӧ����ƽ��״̬������ͼ���жϴ���ƽ��״̬�ĵ㣻
��2�����ٸ���v=
����v��NO2����
��ƽ�ⳣ��ָ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ������ͼ����ƽ��ʱ����ֵ�ƽ��Ũ�ȣ�����ƽ�ⳣ������ʽ���㣻
��25minʱ��X��Ũ������Y��Ũ�Ȳ��䣬ֻ��������X��Ũ�ȣ�d����b����¶���ͬ���¶Ȳ���ƽ�ⳣ�����䣻
��3����25minʱ��X��Ũ������Y��Ũ�Ȳ��䣬ֻ��������X��Ũ�ȣ�
�ڱ����¶Ȳ��䣬������С�����������1L����Ӧ������Ũ��������ɫ���ͬʱѹǿ����ƽ��������Ӧ�ƶ�������������Ũ���ֽ��ͣ�����ԭƽ��Ũ�ȴ���ɫ���dz����ԭƽ����ɫ�
 N2O4��g����֪��NO2��Ũ�ȱ仯��N2O4Ũ�ȱ仯����2�����ݴ��жϣ�
N2O4��g����֪��NO2��Ũ�ȱ仯��N2O4Ũ�ȱ仯����2�����ݴ��жϣ����ʵ�Ũ�Ȳ������仯ʱ��ʾ��ѧ��Ӧ����ƽ��״̬������ͼ���жϴ���ƽ��״̬�ĵ㣻
��2�����ٸ���v=
| ��c |
| ��t |
��ƽ�ⳣ��ָ�ﵽƽ��ʱ��������Ũ�ȵĻ�ѧ���������ݵij˻����Ը���Ӧ��Ũ�ȵĻ�ѧ���������ݵij˻����õı�ֵ������ͼ����ƽ��ʱ����ֵ�ƽ��Ũ�ȣ�����ƽ�ⳣ������ʽ���㣻
��25minʱ��X��Ũ������Y��Ũ�Ȳ��䣬ֻ��������X��Ũ�ȣ�d����b����¶���ͬ���¶Ȳ���ƽ�ⳣ�����䣻
��3����25minʱ��X��Ũ������Y��Ũ�Ȳ��䣬ֻ��������X��Ũ�ȣ�
�ڱ����¶Ȳ��䣬������С�����������1L����Ӧ������Ũ��������ɫ���ͬʱѹǿ����ƽ��������Ӧ�ƶ�������������Ũ���ֽ��ͣ�����ԭƽ��Ũ�ȴ���ɫ���dz����ԭƽ����ɫ�
����⣺��1����ͼ��֪10-25minƽ��״̬ʱ��X��ʾ���������Ũ�ȱ仯��Ϊ��0.6-0.2��mol/L=0.4mol/L��Y��ʾ�ķ�Ӧ���Ũ�ȱ仯��Ϊ��0.6-0.4��mol/L=0.2mol/L��X��ʾ���������Ũ�ȱ仯����Y��ʾ�ķ�Ӧ���Ũ�ȱ仯����2��������X��ʾNO2Ũ����ʱ��ı仯���ߣ�Y��ʾN2O4Ũ����ʱ��ı仯���ߣ�
��ͼ��֪��10-25min��35min֮��X��Y�����ʵ����������仯������Ӧʱ����ڵĵ㴦�ڻ�ѧƽ��״̬����b��d���ڻ�ѧƽ��״̬��
�ʴ�Ϊ��X��b��d��
��2����X��ʾNO2Ũ����ʱ��ı仯���ߣ�Y��ʾN2O4Ũ����ʱ��ı仯���ߣ���ͼ��֪��ǰ10min�ڣ�NO2��Ũ�ȱ仯��Ϊ��0.6-0.2��mol/L=0.4mol/L�����Ԧԣ�NO2��=
=0.04mol?L-1?min-1���ʴ�Ϊ��0.04mol?L-1?min-1��
����ͼ��֪��b��ʱ������ƽ��Ũ��Ϊc��NO2��=0.6mol/L��c��N2O4��=0.4mol/L������b���ƽ�ⳣ��K��b��=
=
���ʴ�Ϊ��
��
��25minʱ˲�䣬X��Ũ������Y��Ũ�Ȳ��䣬ֻ��������X��Ũ�ȣ�d����b����¶���ͬ��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ���ƽ�ⳣ�����䣬��K��d��=K��b����
�ʴ�Ϊ��=��
��3������25minʱ��X��Ũ������Y��Ũ�Ȳ��䣬ֻ��������X��Ũ�ȣ�������NO2 ��Ũ�ȣ���ѡ��C��
�ڱ����¶Ȳ��䣬������С�����������1L����Ӧ������Ũ��������ɫ���ͬʱѹǿ����ƽ��������Ӧ�ƶ�������������Ũ���ֽ��ͣ�����ԭƽ��Ũ�ȴ���ɫ���dz����ԭƽ����ɫ��ʴ�Ϊ���ȱ�����dz������ԭƽ����ɫ�
��ͼ��֪��10-25min��35min֮��X��Y�����ʵ����������仯������Ӧʱ����ڵĵ㴦�ڻ�ѧƽ��״̬����b��d���ڻ�ѧƽ��״̬��
�ʴ�Ϊ��X��b��d��
��2����X��ʾNO2Ũ����ʱ��ı仯���ߣ�Y��ʾN2O4Ũ����ʱ��ı仯���ߣ���ͼ��֪��ǰ10min�ڣ�NO2��Ũ�ȱ仯��Ϊ��0.6-0.2��mol/L=0.4mol/L�����Ԧԣ�NO2��=
| 0.4mol/L |
| 10min |
����ͼ��֪��b��ʱ������ƽ��Ũ��Ϊc��NO2��=0.6mol/L��c��N2O4��=0.4mol/L������b���ƽ�ⳣ��K��b��=
| 0.4 |
| 0.62 |
| 10 |
| 9 |
| 10 |
| 9 |
��25minʱ˲�䣬X��Ũ������Y��Ũ�Ȳ��䣬ֻ��������X��Ũ�ȣ�d����b����¶���ͬ��ƽ�ⳣ��ֻ���¶�Ӱ�죬�¶Ȳ���ƽ�ⳣ�����䣬��K��d��=K��b����
�ʴ�Ϊ��=��
��3������25minʱ��X��Ũ������Y��Ũ�Ȳ��䣬ֻ��������X��Ũ�ȣ�������NO2 ��Ũ�ȣ���ѡ��C��
�ڱ����¶Ȳ��䣬������С�����������1L����Ӧ������Ũ��������ɫ���ͬʱѹǿ����ƽ��������Ӧ�ƶ�������������Ũ���ֽ��ͣ�����ԭƽ��Ũ�ȴ���ɫ���dz����ԭƽ����ɫ��ʴ�Ϊ���ȱ�����dz������ԭƽ����ɫ�
���������⿼��ƽ�ⳣ����ƽ���ƶ���ƽ��Ӱ�����ء���ѧƽ��ͼ��ȣ��Ѷ��еȣ�ͼ��������ⲽ�裺��1������ͼ�ٿ��棨��Ū�������������������壩���ڿ��ߣ���Ū���ߵ�����ͱ仯���ƣ����ۿ��㣨��Ū����㡢�յ㡢���㡢�յ�����壩���ܿ��Ƿ�Ҫ�������ߣ�������ߡ���ѹ�ߣ����ݿ�����ͼ�����й����Ķ��٣���2��������ɣ�������������ĸı�Ի�ѧ��Ӧ���ʺͻ�ѧƽ���Ӱ����ɣ�

��ϰ��ϵ�д�
�����Ŀ
 ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ں��������½�һ����NO2��N2O4�Ļ������ͨ��һ�ݻ�Ϊ2L���ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵����ȷ���ǣ�������
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ں��������½�һ����NO2��N2O4�Ļ������ͨ��һ�ݻ�Ϊ2L���ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵����ȷ���ǣ�������| A��ͼ�е��������ߣ�X�DZ�ʾNO2Ũ����ʱ��ı仯���� | B��a��b��c��d�ĸ����У�ֻ��b��d��Ļ�ѧ��Ӧ����ƽ��״̬ | C��25 minʱ������ƽ���ƶ���ԭ���ǽ��ܱ������������СΪ1L | D��ǰ10 min����v��NO2����ʾ�Ļ�ѧ��Ӧ����Ϊ0.06 mol/��L?min�� |
 ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ��
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ��
 ��
�� ��ʵ����N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽ
��ʵ����N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽ ��2009?��Ǩ��ģ����֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ں��������½�һ����NO2��N2O4�Ļ������ͨ��һ�ݻ�Ϊ2L���ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵����ȷ���ǣ�������
��2009?��Ǩ��ģ����֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ں��������½�һ����NO2��N2O4�Ļ������ͨ��һ�ݻ�Ϊ2L���ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵����ȷ���ǣ������� ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g��������ӦΪ���ȷ�Ӧ�����ֽ�һ����NO2 ��N2O4 �Ļ������ͨ��һ���Ϊ1L�ĺ����ܱ������У���Ӧ���Ũ����ʱ��仯�Ĺ�ϵ������ͼ��ʾ����ش��������⣺
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g��������ӦΪ���ȷ�Ӧ�����ֽ�һ����NO2 ��N2O4 �Ļ������ͨ��һ���Ϊ1L�ĺ����ܱ������У���Ӧ���Ũ����ʱ��仯�Ĺ�ϵ������ͼ��ʾ����ش��������⣺