��Ŀ����
 ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ��
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ֽ�һ����NO2��N2O4�Ļ������ͨ��һ�����Ϊ2L�ĺ����ܱղ��������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ��ʾ����1��ͼ�й�����������X��Y����������
X
X
��ʾNO2Ũ����ʱ��ı仯��a��b��c��d�ĸ����У���ʾ��ѧ��Ӧ����ƽ��״̬�ĵ���bd
bd
�����в���˵���÷�Ӧ�Ѵﵽƽ��״̬����B
B
��A�������ڻ�������ѹǿ����ʱ��仯���ı�
B�������ڻ��������ܶȲ���ʱ��仯���ı�
C�������ڻ���������ɫ����ʱ��仯���ı�
D�������ڻ�������ƽ����Է�����������ʱ��仯���ı�
��2����ǰ10min����NO2��ʾ�Ļ�ѧ��Ӧ����v��NO2��=
0.04
0.04
mol/��L?min������0��15min����Ӧ2NO2��g��?N2O4��g����ƽ�ⳣ��K��b��=
| 10 |
| 9 |
| 10 |
| 9 |
��3����Ӧ25minʱ����ֻ�ı���ijһ��������ʹ���߷�������ͼ��ʾ�ı仯��������������
����NO2��Ũ��
����NO2��Ũ��
�������ֱ������ƽ�ⳣ��K��d��=
=
K��b�����������=������������4����Ҫ�ﵽʹNO2��g���İٷֺ�����d����ͬ�Ļ�ѧƽ��״̬����25minʱ�����Բ�ȡ�Ĵ�ʩ��
B��D
B��D
��A���������������B�����������
C�������¶� D������һ������N2O4��
��������1�����ݷ�Ӧ����ʽ2NO2��g�� N2O4��g����֪��NO2��Ũ�ȱ仯��N2O4Ũ�ȱ仯����2�����ݴ˽��ͼ��������Ũ�ȵı仯���жϣ�
N2O4��g����֪��NO2��Ũ�ȱ仯��N2O4Ũ�ȱ仯����2�����ݴ˽��ͼ��������Ũ�ȵı仯���жϣ�
���ʵ�Ũ�Ȳ������仯ʱ��ʾ��ѧ��Ӧ����ƽ��״̬������ͼ���жϴ���ƽ��״̬�ĵ㣻�����ڻ�������������䣬�������������ܶȲ��䣻
��2���ٸ���v=
����v��NO2����
��0��15min������ͼ�����ƽ��ʱ��Ũ�ȣ��ٸ���K�ı���ʽ���K��
��3�������߿���25 minʱ��NO2��Ũ��ͻȻ�����¶Ȳ��䣬��ѧƽ�ⳣ�����䣻
��4������ѹǿ��ƽ���Ӱ�������
 N2O4��g����֪��NO2��Ũ�ȱ仯��N2O4Ũ�ȱ仯����2�����ݴ˽��ͼ��������Ũ�ȵı仯���жϣ�
N2O4��g����֪��NO2��Ũ�ȱ仯��N2O4Ũ�ȱ仯����2�����ݴ˽��ͼ��������Ũ�ȵı仯���жϣ����ʵ�Ũ�Ȳ������仯ʱ��ʾ��ѧ��Ӧ����ƽ��״̬������ͼ���жϴ���ƽ��״̬�ĵ㣻�����ڻ�������������䣬�������������ܶȲ��䣻
��2���ٸ���v=
| ��c |
| ��t |
��0��15min������ͼ�����ƽ��ʱ��Ũ�ȣ��ٸ���K�ı���ʽ���K��
��3�������߿���25 minʱ��NO2��Ũ��ͻȻ�����¶Ȳ��䣬��ѧƽ�ⳣ�����䣻
��4������ѹǿ��ƽ���Ӱ�������
����⣺��1����ͼ��֪10-25minƽ��״̬ʱ��X��ʾ���������Ũ�ȱ仯��Ϊ��0.6-0.2��mol/L=0.4mol/L��Y��ʾ�ķ�Ӧ���Ũ�ȱ仯��Ϊ��0.6-0.4��mol/L=0.2mol/L��X��ʾ���������Ũ�ȱ仯����Y��ʾ�ķ�Ӧ���Ũ�ȱ仯����2��������X��ʾNO2Ũ����ʱ��ı仯���ߣ�Y��ʾN2O4Ũ����ʱ��ı仯���ߣ�
��ͼ��֪��10-25min��30min֮��X��Y�����ʵ����������仯������Ӧʱ����ڵĵ㴦�ڻ�ѧƽ��״̬����b��d���ڻ�ѧƽ��״̬�������ڻ�������������䣬�������������ܶȲ��䣬��ˣ��ܶȲ����ж�ƽ�⣻
�ʴ�Ϊ��X��bd��B��
��2����X��ʾNO2Ũ����ʱ��ı仯���ߣ�Y��ʾN2O4Ũ����ʱ��ı仯���ߣ���ͼ��֪��ǰ10min�ڣ�NO2��Ũ�ȱ仯��Ϊ��0.6-0.2��mol/L=0.4mol/L�����Ԧԣ�NO2��=
=0.04mol?L-1?min-1��
��0��15min����Ӧ2NO2��g��?N2O4��g����v��NO2��=0.6mol?L-1��v��N2O4��=0.4mol?L-1����K=
=
=
���ʴ�Ϊ��
��
��3�������߿���25 minʱ��NO2��Ũ��ͻȻ����֪�ı������Ϊ����NO2��Ũ�ȣ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣻�ʴ�Ϊ������NO2��Ũ�ȣ�=��
��4������25 minʱ��������NO2��Ũ�ȣ��൱��������ѹǿ��ƽ�����ƣ�d��ʱNO2�İٷֺ���С��bʱNO2�ٷֺ�����Ҫʹ25 minʱ�ı������ﵽʹNO2��g���İٷֺ�����d����ͬ�Ļ�ѧƽ��״̬���ɼ�ѹ������һ������N2O4��Ҳ�൱�ڼ�ѹ���ʴ�Ϊ��B��D��
��ͼ��֪��10-25min��30min֮��X��Y�����ʵ����������仯������Ӧʱ����ڵĵ㴦�ڻ�ѧƽ��״̬����b��d���ڻ�ѧƽ��״̬�������ڻ�������������䣬�������������ܶȲ��䣬��ˣ��ܶȲ����ж�ƽ�⣻
�ʴ�Ϊ��X��bd��B��
��2����X��ʾNO2Ũ����ʱ��ı仯���ߣ�Y��ʾN2O4Ũ����ʱ��ı仯���ߣ���ͼ��֪��ǰ10min�ڣ�NO2��Ũ�ȱ仯��Ϊ��0.6-0.2��mol/L=0.4mol/L�����Ԧԣ�NO2��=
| 0.4mol/L |
| 10min |
��0��15min����Ӧ2NO2��g��?N2O4��g����v��NO2��=0.6mol?L-1��v��N2O4��=0.4mol?L-1����K=
| v(N2O4) |
| v2(NO2) |
| 0.4 |
| 0��62 |
| 10 |
| 9 |
| 10 |
| 9 |
��3�������߿���25 minʱ��NO2��Ũ��ͻȻ����֪�ı������Ϊ����NO2��Ũ�ȣ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣻�ʴ�Ϊ������NO2��Ũ�ȣ�=��
��4������25 minʱ��������NO2��Ũ�ȣ��൱��������ѹǿ��ƽ�����ƣ�d��ʱNO2�İٷֺ���С��bʱNO2�ٷֺ�����Ҫʹ25 minʱ�ı������ﵽʹNO2��g���İٷֺ�����d����ͬ�Ļ�ѧƽ��״̬���ɼ�ѹ������һ������N2O4��Ҳ�൱�ڼ�ѹ���ʴ�Ϊ��B��D��
���������⿼��ı���������Ի�ѧ��Ӧ���ʼ���ѧƽ���Ӱ�졢��ѧƽ��ͼ��Ӧ���ʼ���ȣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
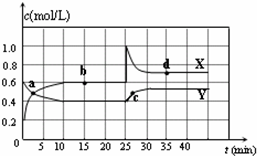 ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ں��������½�һ����NO2��N2O4�Ļ������ͨ��һ�ݻ�Ϊ2L���ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵����ȷ���ǣ�������
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ں��������½�һ����NO2��N2O4�Ļ������ͨ��һ�ݻ�Ϊ2L���ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵����ȷ���ǣ�������| A��ͼ�е��������ߣ�X�DZ�ʾNO2Ũ����ʱ��ı仯���� | B��a��b��c��d�ĸ����У�ֻ��b��d��Ļ�ѧ��Ӧ����ƽ��״̬ | C��25 minʱ������ƽ���ƶ���ԭ���ǽ��ܱ������������СΪ1L | D��ǰ10 min����v��NO2����ʾ�Ļ�ѧ��Ӧ����Ϊ0.06 mol/��L?min�� |
 ��
�� ��ʵ����N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽ
��ʵ����N-N������Ϊ167kJ?mol-1��NO2�е������ļ���Ϊ466kJ?mol-1��N2O4�е������ļ���Ϊ438.5kJ?mol-1����д��NO2ת��ΪN2O4���Ȼ�ѧ����ʽ ��2009?��Ǩ��ģ����֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ں��������½�һ����NO2��N2O4�Ļ������ͨ��һ�ݻ�Ϊ2L���ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵����ȷ���ǣ�������
��2009?��Ǩ��ģ����֪NO2��N2O4�����ת����2NO2��g��?N2O4��g����H��0���ں��������½�һ����NO2��N2O4�Ļ������ͨ��һ�ݻ�Ϊ2L���ܱ������У���Ӧ��Ũ����ʱ��仯��ϵ��ͼ������˵����ȷ���ǣ������� ��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g��������ӦΪ���ȷ�Ӧ�����ֽ�һ����NO2 ��N2O4 �Ļ������ͨ��һ���Ϊ1L�ĺ����ܱ������У���Ӧ���Ũ����ʱ��仯�Ĺ�ϵ������ͼ��ʾ����ش��������⣺
��֪NO2��N2O4�����ת����2NO2��g��?N2O4��g��������ӦΪ���ȷ�Ӧ�����ֽ�һ����NO2 ��N2O4 �Ļ������ͨ��һ���Ϊ1L�ĺ����ܱ������У���Ӧ���Ũ����ʱ��仯�Ĺ�ϵ������ͼ��ʾ����ش��������⣺