��Ŀ����
�����£�0.1mol/L�İ�ˮpH��11�����������������
c(NH3��H2O)��c(Cl��)��c(NH+)��c(OH��)��c(H+)
| A�������Һ�м�ˮϡ�ͣ�c(OH��)/c(NH3��H2O )���� |
| B��0.lmol/L��ˮ��0.lmol/LH2SO4��Һ�������Ϻ�������Һ�У�c(NH4+)+c(H+)��2c(SO42��)+c(OH��) |
| C��0.1mol/L��ˮ��0.05mol/LHCl��Һ�������Ϻ�������Һ�У�c(NH4+)+n(NH3)+n(NH3��H2O)��2n(Cl��) |
| D��Ũ�Ⱦ�Ϊ0.1mol/L��ˮ��NH4Cl��Һ�������Ϻ�����Һ�ʼ��ԣ��� |
D
����������ڰ�ˮ�д���ƽ�⣺NH3��H2O
 NH4++OH-�������Һ�м�ˮϡ�ͣ�c(OH��)��С�� c(NH3��H2O )Ҳ��С����������ƽ�������ƶ�������c(NH3��H2O )��С�ı�������c(OH��)��С�ı���������c(OH��)/c(NH3��H2O )������ȷ��B��0.lmol/L��ˮ��0.lmol/LH2SO4��Һ�������Ϻ�������Һ�����ݵ���غ�ɵã�c(NH4+)+c(H+)��2c(SO42��)+c(OH��)����ȷ��C��0.1mol/L��ˮ��0.05mol/LHCl��Һ�������Ϻ�������Һ�����������غ�ɵã�n(NH4+)+n(NH3)+n(NH3��H2O)��2n(Cl��)����ȷ��D��Ũ�Ⱦ�Ϊ0.1mol/L��ˮ��NH4Cl��Һ�������Ϻ�����Һ�ʼ��ԣ���NH3��H2O�ĵ������ô���NH4+��ˮ�����ã�c(NH4+)��c(Cl��)��c(NH3��H2O)��c(OH��)��c(H+)������
NH4++OH-�������Һ�м�ˮϡ�ͣ�c(OH��)��С�� c(NH3��H2O )Ҳ��С����������ƽ�������ƶ�������c(NH3��H2O )��С�ı�������c(OH��)��С�ı���������c(OH��)/c(NH3��H2O )������ȷ��B��0.lmol/L��ˮ��0.lmol/LH2SO4��Һ�������Ϻ�������Һ�����ݵ���غ�ɵã�c(NH4+)+c(H+)��2c(SO42��)+c(OH��)����ȷ��C��0.1mol/L��ˮ��0.05mol/LHCl��Һ�������Ϻ�������Һ�����������غ�ɵã�n(NH4+)+n(NH3)+n(NH3��H2O)��2n(Cl��)����ȷ��D��Ũ�Ⱦ�Ϊ0.1mol/L��ˮ��NH4Cl��Һ�������Ϻ�����Һ�ʼ��ԣ���NH3��H2O�ĵ������ô���NH4+��ˮ�����ã�c(NH4+)��c(Cl��)��c(NH3��H2O)��c(OH��)��c(H+)������
��ϰ��ϵ�д�
�����Ŀ
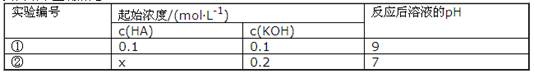
 ��L��1
��L��1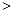 0��1mol��L-l
0��1mol��L-l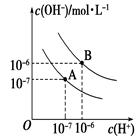
 L-1H2SO4ϴ��Fe�ۣ���Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��֮��������ˮϴ�����������ԣ�
L-1H2SO4ϴ��Fe�ۣ���Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��֮��������ˮϴ�����������ԣ� Na2CO3 + 2HClO
Na2CO3 + 2HClO