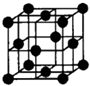
��Ŀ����
��֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������A��B��C��ͬһ���ڵķǽ���Ԫ�أ�������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��AC2Ϊ�Ǽ��Է��ӣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ�E��ԭ������Ϊ24��ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磮���������������ش��������⣺������ʱ��A��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ
��2��B���⻯��ķ��ӿռ乹����
��3��д��������AC2�ĵ���ʽ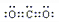
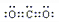 ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�仯ѧʽΪ
��4��ECl3�γɵ������Ļ�ѧʽΪ
��5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
��1��A��B��C�ĵ�һ��������С�����˳��Ϊ
C��O��N
C��O��N
����2��B���⻯��ķ��ӿռ乹����
������
������
��������ԭ�Ӳ�ȡsp3
sp3
�ӻ�����3��д��������AC2�ĵ���ʽ
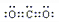
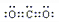
N2O
N2O
����4��ECl3�γɵ������Ļ�ѧʽΪ
[Cr��NH3��4��H2O��2]Cl3
[Cr��NH3��4��H2O��2]Cl3
����5��B������������Ӧ��ˮ�����ϡ��Һ��D�ĵ��ʷ�Ӧʱ��B����ԭ����ͼۣ��÷�Ӧ�Ļ�ѧ����ʽ��
4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O
4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O
��������������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��D��C����һ���ڣ�DӦΪMgԪ�أ�CΪOԪ�أ�AC2Ϊ�Ǽ��Է��ӣ�ӦΪCO2����AΪCԪ�أ���BΪNԪ�أ�E��ԭ������Ϊ24��ӦΪCrԪ�أ���϶�ӦԪ�صĵ��ʺͻ�����������Լ���ĿҪ��ɽ����⣮
����⣺������DCΪ���ӻ����D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��˵��D��C����һ���ڣ�DӦΪMgԪ�أ�CΪOԪ�أ�AC2Ϊ�Ǽ��Է��ӣ�ӦΪCO2����AΪCԪ�أ���BΪNԪ�أ�E��ԭ������Ϊ24��ӦΪCrԪ�أ�
��1��ͬ����Ԫ�ص�һ�����ܴ���������������ƣ��ʵ����ܴ�СΪC��O��N���ʴ�Ϊ��C��O��N��
��2��BΪNԪ�أ���Ӧ���⻯��ΪNH3������������ԭ���γ�3���ļ�����1���µ��Ӷԣ�Ϊsp3�ӻ����ռ乹��Ϊ�����Σ��ʴ�Ϊ�������Σ�sp3��
��3��AC2ΪCO2��Ϊ���ۻ��������ʽΪ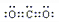 ��һ����B��C��ɵĻ�������CO2��Ϊ�ȵ����壬�ȵ�����ָ��������ԭ������ͬ�ķ��ӡ����ӻ���ţ��������ΪN2O���ʴ�Ϊ��
��һ����B��C��ɵĻ�������CO2��Ϊ�ȵ����壬�ȵ�����ָ��������ԭ������ͬ�ķ��ӡ����ӻ���ţ��������ΪN2O���ʴ�Ϊ��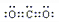 ��N2O��
��N2O��
��4����ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磬��֪�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��[Cr��NH3��4��H2O��2]Cl3��
��5��N����ͼ�Ϊ-3��DΪMgԪ�أ���Ӧ����NH4NO3����Ӧ�ķ���ʽΪ4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
��1��ͬ����Ԫ�ص�һ�����ܴ���������������ƣ��ʵ����ܴ�СΪC��O��N���ʴ�Ϊ��C��O��N��
��2��BΪNԪ�أ���Ӧ���⻯��ΪNH3������������ԭ���γ�3���ļ�����1���µ��Ӷԣ�Ϊsp3�ӻ����ռ乹��Ϊ�����Σ��ʴ�Ϊ�������Σ�sp3��
��3��AC2ΪCO2��Ϊ���ۻ��������ʽΪ
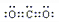 ��һ����B��C��ɵĻ�������CO2��Ϊ�ȵ����壬�ȵ�����ָ��������ԭ������ͬ�ķ��ӡ����ӻ���ţ��������ΪN2O���ʴ�Ϊ��
��һ����B��C��ɵĻ�������CO2��Ϊ�ȵ����壬�ȵ�����ָ��������ԭ������ͬ�ķ��ӡ����ӻ���ţ��������ΪN2O���ʴ�Ϊ��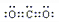 ��N2O��
��N2O����4����ECl3����B��C���⻯���γ�����λ��������������������ʵ���֮��Ϊ2��1������������λ����磬��֪�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��[Cr��NH3��4��H2O��2]Cl3��
��5��N����ͼ�Ϊ-3��DΪMgԪ�أ���Ӧ����NH4NO3����Ӧ�ķ���ʽΪ4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3 +3H2O��
���������⿼��Ԫ���ƶ��⣬������ԭ�ӽṹ��Ԫ�������ɵĿ��飬Ϊ�߿��������ͣ��ƶϳ�Ԫ�ص������ǽ����Ĺؼ����ƶ�ʱע���ԭ�ӵĺ�������Ų��ص��Լ�Ԫ�ص���������Ϊͻ�ƿڽ�𣬱������һ���Ѷȣ�

��ϰ��ϵ�д�
 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�
�����Ŀ
��֪A��B��C��D�ֱ���Cu��Ag��Fe��Al���ֽ����е�һ�֣���֪��A��C������ϡ���ᷴӦ�ų����壻��B��D�������η�Ӧ���û�������D����C��ǿ�Ӧ�ų����壬�ɴ˿����ƶ�A��B��C��D�����ǣ�������
| A��Fe��Cu��Al��Ag | B��Al��Cu��Fe��Ag | C��Cu��Ag��Al��Fe | D��Ag��Al��Cu��Fe |

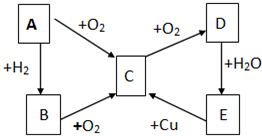 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ� ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�