��Ŀ����
�������γɶ������ӣ���N3-��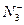 ��
��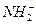 ��
��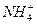 ��
��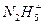 ��
��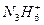 �ȣ���֪
�ȣ���֪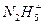 ��
��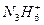 �������Է��ӽ�������γɵģ���������
�������Է��ӽ�������γɵģ���������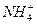 �����ʣ��������Ҳ���γ�NH3��N2H4��N3H5��N4H6��N5H7����
�����ʣ��������Ҳ���γ�NH3��N2H4��N3H5��N4H6��N5H7����
(1)һ��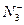 ����__________________________�����ӣ�
����__________________________�����ӣ�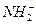 �ĵ���ʽΪ____________��
�ĵ���ʽΪ____________��
(2)�γ�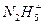 �����Է��ӵĽṹʽ��_____________________��
�����Է��ӵĽṹʽ��_____________________��
(3)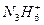 ��ǿ����Һ�з�Ӧ�����ӷ���ʽ��_____________________________��
��ǿ����Һ�з�Ӧ�����ӷ���ʽ��_____________________________��
(4)NH3��N2H4��N3H5��N4H6��N5H7�Ƿ�Ϊͬϵ�Ϊʲô��__________________________��
(5)д��N5H7���ܵĽṹ��ʽ______________________________��
(6)��һϵ�л�������N�������������ֵӦС��____________%��
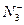 ��
��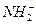 ��
��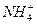 ��
��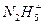 ��
��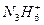 �ȣ���֪
�ȣ���֪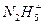 ��
��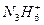 �������Է��ӽ�������γɵģ���������
�������Է��ӽ�������γɵģ���������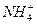 �����ʣ��������Ҳ���γ�NH3��N2H4��N3H5��N4H6��N5H7����
�����ʣ��������Ҳ���γ�NH3��N2H4��N3H5��N4H6��N5H7����(1)һ��
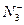 ����__________________________�����ӣ�
����__________________________�����ӣ�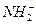 �ĵ���ʽΪ____________��
�ĵ���ʽΪ____________��(2)�γ�
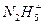 �����Է��ӵĽṹʽ��_____________________��
�����Է��ӵĽṹʽ��_____________________��(3)
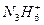 ��ǿ����Һ�з�Ӧ�����ӷ���ʽ��_____________________________��
��ǿ����Һ�з�Ӧ�����ӷ���ʽ��_____________________________��(4)NH3��N2H4��N3H5��N4H6��N5H7�Ƿ�Ϊͬϵ�Ϊʲô��__________________________��
(5)д��N5H7���ܵĽṹ��ʽ______________________________��
(6)��һϵ�л�������N�������������ֵӦС��____________%��
(1)22 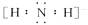 (��1��)
(��1��)
(2)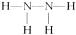 (2��)
(2��)
(3) +OH-====N3H5+H2O(2��)
+OH-====N3H5+H2O(2��)
(4)��Ϊͬϵ���Ϊ���ǵĽṹ���ƣ���������һ�������ɸ���NH��ԭ����(2��)
(5)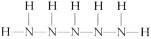 (2��)
(2��)
(6)93.3(2��)
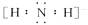 (��1��)
(��1��)(2)
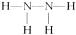 (2��)
(2��)(3)
 +OH-====N3H5+H2O(2��)
+OH-====N3H5+H2O(2��)(4)��Ϊͬϵ���Ϊ���ǵĽṹ���ƣ���������һ�������ɸ���NH��ԭ����(2��)
(5)
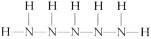 (2��)
(2��)(6)93.3(2��)
�������Ҫ������Ŀ����������ѧ֪ʶ��������Ǩ�ƣ����������ȷ��ϵ��κ�ͬϵ���֪ʶ�����Ȼ��ó�������ۡ���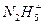 ��
��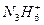 �������Է��ӽ�������γɵģ���������
�������Է��ӽ�������γɵģ���������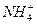 �����ʣ����Կ���ͨ������������ӽ���Ǩ�Ʒ���
�����ʣ����Կ���ͨ������������ӽ���Ǩ�Ʒ���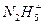 ��
��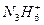 �����ʡ�
�����ʡ�
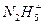 ��
��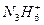 �������Է��ӽ�������γɵģ���������
�������Է��ӽ�������γɵģ���������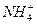 �����ʣ����Կ���ͨ������������ӽ���Ǩ�Ʒ���
�����ʣ����Կ���ͨ������������ӽ���Ǩ�Ʒ���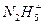 ��
��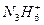 �����ʡ�
�����ʡ�
��ϰ��ϵ�д�
�����Ŀ
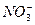 ��Cl-
��Cl-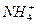 ��K+��OH-��
��K+��OH-��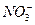 �����ʵ���Ϊ( )
�����ʵ���Ϊ( )