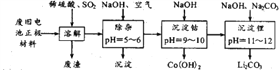
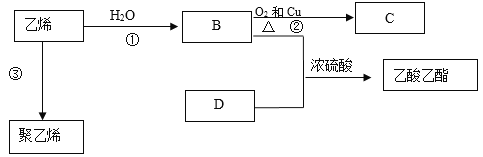
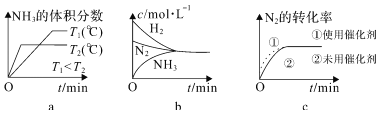
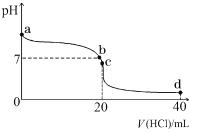
��Ŀ����
����Ŀ��������A��Ԫ���γɵĻ���������Ҫ�İ뵼�������Ӧ����㷺�����黯��(GaAs)���ش��������⣺
��1����̬Gaԭ�ӵĺ�������Ų�ʽΪ__________����̬Asԭ�Ӻ�����__________��δ�ɶԵ��ӡ�
��2����ʧȥ���ӵ�������(��λ��kJ��mol-1)����ֵ����Ϊ577��1985��2962��6192���ɴ˿���֪�ص���Ҫ���ϼ�Ϊ__________��+3����ĵ縺�Ա���__________(������������С��)��
��3���Ƚ������ص�±������۵�ͷе㣬������仯���ɼ�ԭ��__________________________��
�ص�±���� | GaCl3 | GaBr3 | GaI3 |
�۵�/�� | 77.75 | 122.3 | 211.5 |
�е�/�� | 201.2 | 279 | 346 |
GaF3���۵㳬��1000�������ܵ�ԭ����_______________________________________��
��4����ˮ�ϲ����صĽṹ��ͼ��ʾ��������ԭ�ӵ���λ��Ϊ__________���������̼ԭ�ӵ��ӻ���ʽΪ__________��

��5���黯���۵�Ϊ1238�������������ṹ��ͼ��ʾ����������Ϊa=565pm���þ��������Ϊ__________��������ܶ�Ϊ__________(��NAΪ�����ӵ���������ֵ���г���ʽ����)g��cm-3��

���𰸡� [ Ar ]3d104s24p1(��1s22s22p63s23p63d104s24p1) 3 +1(д+1��+2) �� GaCl3��GaBr3��GaI3���ۡ��е��������ߡ����Ǿ�Ϊ���Ӿ������ṹ��������Է��������������������Ӽ�������������ǿ GaF3Ϊ���Ӿ��� 4 Sp2 ԭ�Ӿ��� 
��������(1) Ga��ԭ������Ϊ31�����Ի�̬ԭ�ӵĵ����Ų�ʽΪ[ Ar ]3d104s24p1(��1s22s22p6 3s23p6 3d104s24p1)��As��ԭ������Ϊ33�����̬ԭ�ӵĵ����Ų�ʽΪ[ Ar ]3d104s24p3�����Ի�̬Asԭ�Ӻ�����3��δ�ɶԵ��ӣ�
(2)����������̬ԭ��ʧȥ��������Ҫ�����������ص�ǰ�ļ������ܿ�֪������Ҫ���ϼ�Ϊ+1��+3������As�����������Ų�Ϊ4s24p3����ȫ�����������Ga�����������Ų�Ϊ4s24p1���ر���4p1��ʧ������������ĵ縺�Ա��ش�
(3)����������ʾ���ص�±������۵�ͷе㶼���ߣ��Ұ����ȡ��塢���������ߣ�ԭ�������ǵ������ͬ���ṹ���ƣ����Ƿ��Ӿ��壬����������Է��������������Ӽ������������۷е����ߣ���GaF3���۵㳬��1000����������F�ĵ縺�Ժܴ��γɵ�GaF3�����Ӿ��壻
(4)�ɶ�ˮ�ϲ����صĽṹͼ�ɵã���ԭ�ӵ���λ��Ϊ4���������̼ԭ�����Ȼ��е�̼ԭ�ӵ��ӻ���ʽ��ͬ���γɵĶ���ƽ��ṹ������Ӧ����sp2�ӻ���
(5)���ڸþ�����۵�ߣ�������ض����ǻ���Ԫ�أ����Ըþ�����ԭ�Ӿ��壬�仯ѧʽΪGa4As4���þ���������m=![]() g�����ΪV =(565��10-10)3 cm3�������ܶ�Ϊ
g�����ΪV =(565��10-10)3 cm3�������ܶ�Ϊ![]() g/cm3��
g/cm3��

 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�