题目内容
【题目】根据能量变化示意图,下列热化学方程式正确的是( )
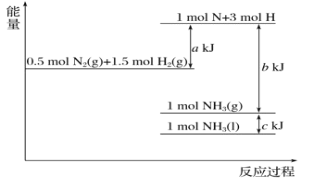
A.N2(g)+3H2(g)=2NH3(g) ΔH=-(b-a)kJ/mol
B.N2(g)+3H2(g)=2NH3(g) ΔH=-(a-b)kJ/mol
C.2NH3(l)=N2(g)+3H2(g) ΔH=2(b+c-a)kJ/mol
D.2NH3(l)=N2(g)+3H2(g) ΔH=2(a+b-c)kJ/mol
【答案】C
【解析】
焓变等于反应物断裂化学键吸收的能量减去形成化学键释放的能量,由图可知,![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g) △H=(a-b)kJmol-1,
H2(g)=NH3(g) △H=(a-b)kJmol-1,![]() N2(g)+
N2(g)+![]() H2(g)=NH3(l) △H=(a-b-c)kJmol-1,据此分析判断。
H2(g)=NH3(l) △H=(a-b-c)kJmol-1,据此分析判断。
A.![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g) △H=(a-b)kJmol-1,则N2(g)+3H2(g)=2NH3(g) △H=-2(b-a)kJmol-1,故A错误;
H2(g)=NH3(g) △H=(a-b)kJmol-1,则N2(g)+3H2(g)=2NH3(g) △H=-2(b-a)kJmol-1,故A错误;
B.![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g) △H=(a-b)kJmol-1,则N2(g)+3H2(g)=2NH3(g)△H═-2(b-a)kJmol-1,故B错误;
H2(g)=NH3(g) △H=(a-b)kJmol-1,则N2(g)+3H2(g)=2NH3(g)△H═-2(b-a)kJmol-1,故B错误;
C.![]() N2(g)+
N2(g)+![]() H2(g)=NH3(l) △H=(a-b-c)kJmol-1,则N2(g)+3H2(g)=2NH3(l) △H=2(a-b-c)kJmol-1,则2NH3(1)=N2(g)+3H2(g)△H=2(b+c-a)kJmol-1,故C正确;
H2(g)=NH3(l) △H=(a-b-c)kJmol-1,则N2(g)+3H2(g)=2NH3(l) △H=2(a-b-c)kJmol-1,则2NH3(1)=N2(g)+3H2(g)△H=2(b+c-a)kJmol-1,故C正确;
D.![]() N2(g)+
N2(g)+![]() H2(g)=NH3(l) △H=(a-b-c)kJmol-1,则N2(g)+3H2(g)=2NH3(l) △H=2(a-b-c)kJmol-1,则2NH3(1)=N2(g)+3H2(g)△H=2(b+c-a)kJmol-1,故D错误;
H2(g)=NH3(l) △H=(a-b-c)kJmol-1,则N2(g)+3H2(g)=2NH3(l) △H=2(a-b-c)kJmol-1,则2NH3(1)=N2(g)+3H2(g)△H=2(b+c-a)kJmol-1,故D错误;
故选C。

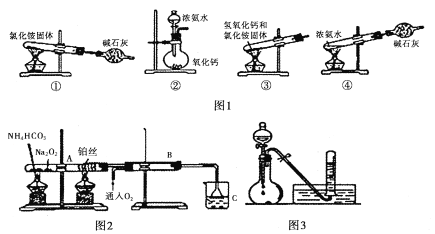
【题目】利用如图所示装置进行下列实验,能得出相应实验结论的是( )
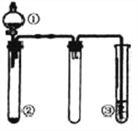
选项 | ① | ② | ③ | 实验结论 |
A | 稀盐酸 | CaCO3 | Na2SiO3溶液 | 非金属性:Cl>C>Si |
B | 浓硫酸 | 蔗糖 | Ba(NO3)2溶液 | 验证SO2与可溶性钡盐可生成白色沉淀 |
C | 浓氨水 | 生石灰 | 酚酞溶液 | 氨气的水溶液呈碱性 |
D | 浓硝酸 | Fe | NaOH溶液 | 铁和浓硝酸反应可生成NO2 |
A. A B. B C. C D. D