��Ŀ����
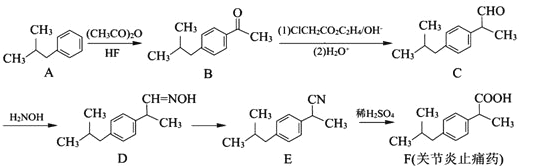
����Ŀ��A��B��C��D��E��F����Ԫ�ؾ�λ�����ڱ���ǰ�����ڣ���ԭ��������������Ԫ��A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵĺ�����ӷ�ռ�ĸ�ԭ�ӹ��(�ܼ�)��DԪ��ԭ�ӵ��ѳɶԵ���������δ�ɶԵ���������3����E��D����ͬһ���壻Fλ��ds������ԭ�ӵ������ֻ��1�����ӡ�
(1)E+���ӵĵ����Ų�ʽ�� ��
(2)B��C��DԪ�صĵ�һ�������ɴ�С��˳���� ��
(3)B��CԪ�ص�ijЩ�⻯��ķ����о�����18�����ӣ���B�������⻯��Ļ�ѧʽ�� ��B��C����Щ�⻯��ķе����ϴ����Ҫԭ���� ��
(4)A��B��D���γɷ���ʽΪA2BD��ij�������û����������Bԭ�ӵĹ���ӻ������� ��1 mol�÷����к�����������Ŀ�� ��

(5)C��F��Ԫ���γɵ�ij������ľ����ṹ��ͼ��ʾ����û�����Ļ�ѧʽ�� ��Cԭ�ӵ���λ���� ��
���𰸡�(1)1s22s22p63s23p63d10����[Ar] 3d10��
(2)N��O��C
(3)C2H6�������⻯��(N2H4)���Ӽ�������
(4)sp2��1 mol��1NA��
(5)Cu3N��6
�����������������A��B��C��D��E����Ԫ�ؾ�λ�����ڱ���ǰ�����ڣ���ԭ��������������Ԫ��A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ���AΪHԪ�أ�B�Ļ�̬ԭ���е���ռ��������������ͬ��ԭ�ӹ����ԭ�Ӻ�������Ų�Ϊ1s22s22p2����BΪ̼Ԫ�أ�Dλ�ڵ�2���ڣ���ԭ�Ӻ���ɶԵ�������δ�ɶԵ�������3����ԭ�Ӻ�������Ų�Ϊ1s22s22p4����DΪOԪ�أ�C��ԭ����������̼����֮�䣬��CΪNԪ�أ�E��D����ͬһ���壬��EΪSԪ�أ�Fλ��ds������ԭ�ӵ������ֻ��1�����ӣ���EΪCu��
(1)ͭΪ29��Ԫ�أ�Cu+���ӵĵ����Ų�ʽΪ1s22s22p63s23p63d10���ʴ�Ϊ��1s22s22p63s23p63d10��
(2)ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���NԪ��2p�ܼ�Ϊ�����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N��O��C���ʴ�Ϊ��N��O��C��
(3)C��N��Ԫ�ؾ����γɺ�18�������⻯����ӣ����⻯����ӷֱ���X��Y��ʾ����XΪC2H6��YΪN2H4��N2H4����֮����������C2H6����֮������������߷е����ϴʴ�Ϊ��C2H6��N2H4����֮����������C2H6����֮���������
(4)A��B��D���γɷ���ʽΪA2BD��ij������ΪH2CO���ṹ��ʽΪHCHO��������Cԭ�ӵļ۲���Ӷ���=3+![]() ��(4-2��2-2)=3���ӻ�����Ϊsp2��1�������к���1��1������1mol�÷����к�����������ĿΪ1NA���ʴ�Ϊ��1NA��
��(4-2��2-2)=3���ӻ�����Ϊsp2��1�������к���1��1������1mol�÷����к�����������ĿΪ1NA���ʴ�Ϊ��1NA��
(5)ԭ��Ϊ�о��������ڵ�ԭ�Ӵ��������ģ�ÿ������Ϊ8���������ã�������ԭ��Ϊ4���������ã���Cԭ����λ��Ϊ![]() =6���ʴ�Ϊ��Cu3N��6��
=6���ʴ�Ϊ��Cu3N��6��

 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�����Ŀ������ʪ����п������ͭ�������������ӵ��������£�

��֪��ͭ������Ҫ��п��ͭ�������ӣ�Cd�����ܣ�Co���ȵ��ʡ�
�±��г�����ؽ������������������������pH����ʼ������pH����������Ũ��Ϊ0.1 mol��L-1���㣩��
�������� | Fe(OH)3 | Fe(OH)2 | Cd(OH)2 | Mn(OH)2 |
��ʼ������pH | 1.5 | 6.5 | 7.2 | 8.2 |
������ȫ��pH | 3.3 | 9.9 | 9.5 | 10.6 |
��1��Ϊ�����ͭ�������������ʣ��ɲ�ȡ�Ĵ�ʩ�У����ʵ������¶ȣ��ڽ��裻��______�ȡ���֪�����Ľ������Ӿ�Ϊ���ۣ�д�������ܵĻ�ѧ����ʽ__________________��
��2�����ܵĹ����У���Ҫ������Sb2O3��п�ۻ���Sb2O3��Co2+����Һ�γ���ز������Ͻ�CoSb������ص�������ӦʽΪ________________________________��
��3�������Ĺ��̷��������У�
���ȼ�������KMnO4��������Ӧ�����ӷ���ʽΪ______________________________��
���ټ���ZnO���Ʒ�ӦҺ��pH��ΧΪ_____________��
��4���������̵ڢٲ���������KMnO4ʱ�������Ʋ�����MnO2�������Ӧ����ɵĽ����______________���������KMnO4����������������Һ����FeԪ�ز��ࡣ�����ʵ�鷽��������֤_________________��
��5�����������Һ�ö��Ե缫���ɻ���ӵ��ʡ�����Һ�п�ѭ�����õ�������____��
��6���������ӷ�ˮ���û�ѧ�������������Ǽ����ӵ����ܻ�������ܶȻ�������25������
Ksp(CdCO3)��5.210��12�� Ksp(CdS)��3.610��29��Ksp(Cd(OH)2)��2.010��16������������Ϣ��
����Cd2+Ч����ѵ��Լ���____________��
a��Na2CO3 b��Na2S c��CaO
��������ʯ�Ҵ������ӷ�ˮ���pHΪ11����ʱ��Һ��c(Cd2+)��_________��