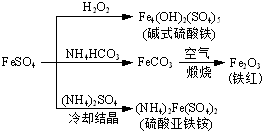
��Ŀ����
��������ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ�γ�ģ������ݣ�����ѡ������һ�������������ȫ������A�����֡�

A����12�֣������ʽṹ�����ʡ�
1915��ŵ��������ѧ������Henry Bragg��Lawrence Bragg���Ա���������X���߶Ծ���ṹ�ķ��������Ĺ��ס�
�ſ�ѧ��ͨ��X����̽����NaCl��KCl��MgO��CaO����ṹ���ƣ��������־��徧�����������±���
4�־���NaCl��KCl��MgO��CaO�۵��ɸߵ��͵�˳���� �� ��
�ƿ�ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�������λ����������������߱�ʾ��
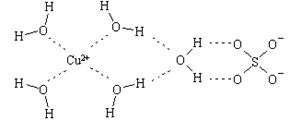
��д����̬Cuԭ�ӵĺ�������Ų�ʽ �� ������ͭ�������� �� ������ĸ���ţ��ѻ���ʽ��
A B C D
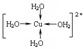
��д������������ˮ��ͭ���ӵĽṹ��ʽ�����뽫��λ����ʾ������ �� ��
��ˮ���Ӽ��������������о�������ʵ˵�������ˮ�����ʵ�Ӱ�� �� ��
��SO42���Ŀռ乹���� �� ��
B����12�֣���ʵ�黯ѧ��
��������淋Ļ�ѧʽΪ(NH4)2SO4��FeSO4��6H2O������Ī���Σ��Ƿ�����ѧ�г����Ļ�ԭ����ij��ѧ�о�С���������ʵ�����Ʊ�Ī���β��ⶨ��������淋Ĵ��ȡ�
����һ����м�Ĵ������������ʢ��������м����ƿ�м���Na2CO3��Һ�����ȣ����ˡ�ϴ�ӡ����������������Ϊm1��
�������FeSO4���Ʊ�����������м���뵽һ������ϡH2SO4�У���ַ�Ӧ����˲���������ˮϴ����ƿ����ֽ����Һ��ϴ��Һ��ȫת�����������С������������أ�������Ϊm2��
����������������淋��Ʊ���ȷ��ȡ����������(NH4)2SO4���롰��������е��������У���������һ��ʱ���ֹͣ����ȴ������������什ᾧ����ˡ���������ˮ�Ҵ�ϴ�Ӳ���Ȼ����������þ���������
�����ģ��ñ�ɫ���ⶨ��������淋Ĵ��ȡ�
�ش��������⣺
�Ų������г�ȡ��(NH4)2SO4����Ϊ �� ��
�Ƣ���м��Na2CO3��Һ������Ŀ���� �� ���Ʊ�FeSO4��Һʱ������ͼװ�ó��ȹ��ˣ�ԭ���� �� ��
�ڽ�(NH4)2SO4��FeSO4��Ϻ���ȡ�Ũ����ֹͣ���ȵ�ʱ���� �� ��
�۱�ɫ���ⶨ��������林��ȵ�ʵ�鲽��Ϊ��Fe3����ɫ�����ơ����������������Һ�����ơ���ɫ�ⶨ����ɫ�ʹ���Һ����ʱ�������������ϡHCl��Һ�⣬��Ӧע��������� �� ��
�ܸ�ʵ������ͨ�� �� ȷ����������鱗�Ʒ�ȼ���

A����12�֣������ʽṹ�����ʡ�
1915��ŵ��������ѧ������Henry Bragg��Lawrence Bragg���Ա���������X���߶Ծ���ṹ�ķ��������Ĺ��ס�
�ſ�ѧ��ͨ��X����̽����NaCl��KCl��MgO��CaO����ṹ���ƣ��������־��徧�����������±���
| ���� | NaCl | KCl | CaO |
| �����ܣ�(kJ��mol��1) | 786 | 715 | 3 401 |
�ƿ�ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�������λ����������������߱�ʾ��

��д����̬Cuԭ�ӵĺ�������Ų�ʽ �� ������ͭ�������� �� ������ĸ���ţ��ѻ���ʽ��
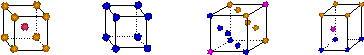 |
A B C D
��д������������ˮ��ͭ���ӵĽṹ��ʽ�����뽫��λ����ʾ������ �� ��
��ˮ���Ӽ��������������о�������ʵ˵�������ˮ�����ʵ�Ӱ�� �� ��
��SO42���Ŀռ乹���� �� ��
B����12�֣���ʵ�黯ѧ��
��������淋Ļ�ѧʽΪ(NH4)2SO4��FeSO4��6H2O������Ī���Σ��Ƿ�����ѧ�г����Ļ�ԭ����ij��ѧ�о�С���������ʵ�����Ʊ�Ī���β��ⶨ��������淋Ĵ��ȡ�
����һ����м�Ĵ������������ʢ��������м����ƿ�м���Na2CO3��Һ�����ȣ����ˡ�ϴ�ӡ����������������Ϊm1��
�������FeSO4���Ʊ�����������м���뵽һ������ϡH2SO4�У���ַ�Ӧ����˲���������ˮϴ����ƿ����ֽ����Һ��ϴ��Һ��ȫת�����������С������������أ�������Ϊm2��
����������������淋��Ʊ���ȷ��ȡ����������(NH4)2SO4���롰��������е��������У���������һ��ʱ���ֹͣ����ȴ������������什ᾧ����ˡ���������ˮ�Ҵ�ϴ�Ӳ���Ȼ����������þ���������
�����ģ��ñ�ɫ���ⶨ��������淋Ĵ��ȡ�
�ش��������⣺
�Ų������г�ȡ��(NH4)2SO4����Ϊ �� ��
�Ƣ���м��Na2CO3��Һ������Ŀ���� �� ���Ʊ�FeSO4��Һʱ������ͼװ�ó��ȹ��ˣ�ԭ���� �� ��
�ڽ�(NH4)2SO4��FeSO4��Ϻ���ȡ�Ũ����ֹͣ���ȵ�ʱ���� �� ��
�۱�ɫ���ⶨ��������林��ȵ�ʵ�鲽��Ϊ��Fe3����ɫ�����ơ����������������Һ�����ơ���ɫ�ⶨ����ɫ�ʹ���Һ����ʱ�������������ϡHCl��Һ�⣬��Ӧע��������� �� ��
�ܸ�ʵ������ͨ�� �� ȷ����������鱗�Ʒ�ȼ���
A����MgO��CaO��NaCl��KCl
�Ƣ�1s22s22p63s23p63d104s1��[Ar] 3d104s1 C
��
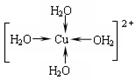
��ˮ���ۡ��е�ϸߣ����ʱ�ܶȼ�С����
����������
 A�������漰����֪ʶ�㡰�����ܡ���������Ų�����λ�����ռ乹�͡�����1���о����ܴ�С���������뾶���ɣ��������ܴ�С��ֱ��Ӱ���۵㣬Mg2+�뾶С��Ca2+��MgO�����ܴ��۵�ߡ�
A�������漰����֪ʶ�㡰�����ܡ���������Ų�����λ�����ռ乹�͡�����1���о����ܴ�С���������뾶���ɣ��������ܴ�С��ֱ��Ӱ���۵㣬Mg2+�뾶С��Ca2+��MgO�����ܴ��۵�ߡ���2������ͭ�Ķѻ���ʽΪC����Ҫ���䣻Cu2+��λ��Ϊ4������Ϊ �����Ӱ����Ӿ��������
���ʱ����۷е㡢�ܶȵȣ�SO42������ԭ��S���ӻ���ʽΪSP3����������λ������Ϊ�������壬���ƿ���˼��PO43����CO32���Ľṹ��Ҳ���ԴӼ۵��ӶԻ��⿼�ǡ�

��ϰ��ϵ�д�
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
�����Ŀ
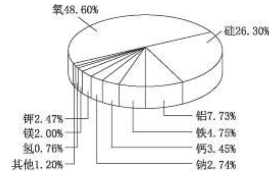
 (C5H5)2 Fe��H2��
(C5H5)2 Fe��H2��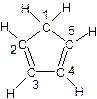 ��ͼ�����ֽ�����̼ԭ�ӱ�ţ�������5��̼ԭ���в�ȡsp3�ӻ����� ����д��ţ���
��ͼ�����ֽ�����̼ԭ�ӱ�ţ�������5��̼ԭ���в�ȡsp3�ӻ����� ����д��ţ���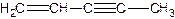 �ǻ�ï��ϩ��һ��ͬ���칹�壬������ӽṹ�д���ͬһƽ���ϵ�ԭ�Ӹ�������� ����
�ǻ�ï��ϩ��һ��ͬ���칹�壬������ӽṹ�д���ͬһƽ���ϵ�ԭ�Ӹ�������� ����