��Ŀ����
14��ij�Ȼ�����Ʒ��������FeCl2���ʣ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�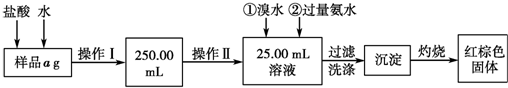
������������̣��ش��������⣺
��1�����������õ��IJ����������ձ����������⣬��������250mL����ƿ����ͷ�ιܣ����������ƣ� ����������õ���������D�������ţ�
A��50mL�ձ� B��50mL��Ͳ C��100mL��Ͳ D��25mL�ζ���
��2����д��������ˮ���������ӷ�Ӧ����ʽ2Fe2++Br2�T2Fe3++2Br-�����백ˮҪ������ԭ����ʹFe3+��ֳ�����
��3����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ�����ȡ���һ��ϴ�ӵ��˳�Һ������һ֧�Թ��У��μ�AgNO3��Һ�����������ɣ���֤������ϴ�Ӹɾ���
��4������������ȣ���ȴ�����£�����ƽ����������Ϊb1 g���ٴμ��Ȳ���ȴ�����³���������Ϊb2 g����b1-b2=0.3g�����������Ӧ���еIJ������ٴμ�����ȴ��������ֱ���������γ�����������С��0.1g��
��5��������ȷ�����ղ����Ľ��ƫ�����������ԭ������ǹ������ʱδ��ַ�Ӧ��ΪFe2O3 ��д��һ��ԭ�ɣ���
��6�������й����ʵ��Ʊ���Ӧ��˵������ȷ����C
A���ڷ�ˮ�е��뱥��FeCl3��Һ���Ʊ�Fe��OH��3����
B�������ȷ�Ӧԭ�����Ƶ��۵�ϸߵĽ�����
C���ú�ˮ��ԭ���Ƶþ��Σ��ٵ�ⴿ���Ȼ�����Һ�õ�������
D��ijЩ���������������Ϊ���ϣ�
���� ��1����������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ�����ݲ���IIΪ�ζ������������õζ��ܣ�
��2������Br2���������ԣ�������Fe2+��Ϊ��ʹFe3+��ֳ�������ˮҪ������
��3������ȡ�������һ��ϴ��Һ���μ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ���
��4��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g��
��5���������������������ʱδ��ַ�Ӧ��ΪFe2O3��
��6��A������FeCl3��Һ�����ˮ���Ʊ�Fe��OH��3���壬�������Ȼ���ˮ����ȴٽ����ɣ�
B�����ȷ�Ӧ����������ǿ��ԭ�Ժͷ��ȷų������ȵ��ص㣻
C����ҵ�����ǵ������NaCl����ȡ��
D��ijЩ����ɫ�������������Ϳ�ϻ��������ɫ��
��� �⣺��1��������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ�������IIΪ�ζ������������õζ��ܣ�
�ʴ𰸣�250mL����ƿ����ͷ�ιܣ�D��
��2����Br2���������ԣ�������Fe2+��2Fe2++Br2=2Fe3++2Br-��Ϊ��ʹFe3+��ֳ�������ˮҪ�������ʴ�Ϊ��2Fe2++Br2=2Fe3++2Br-��ʹFe3+��ֳ�����
��3��ȡ���һ��ϴ��Һ���μ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ�������һ��ϴ��Һ���μ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ���
��4��Ϊ�˼��������ٴμ�����ȴ��������ֱ������������С��0.1g��
�ʴ�Ϊ���ٴμ�����ȴ��������ֱ������������С��0.1g��
��5�������������������ʱδ��ַ�Ӧ��Ϊ Fe2O3���ʴ�Ϊ���������ʱδ��ַ�Ӧ��Ϊ Fe2O3��
��6��A������FeCl3��Һ�����ˮ���Ʊ�Fe��OH��3���壬�������Ȼ���ˮ����ȴٽ����ɣ���������ˮ���йأ���A��ȷ��
B�����ȷ�Ӧ����������ǿ��ԭ�Ժͷ��ȷų������ȵ��ص㣬���Ƶ��۵�ϸߵĽ���������B��ȷ��
C����ҵ�����ǵ������NaCl��2NaCl$\frac{\underline{\;ͨ��\;}}{\;}$2Na+Cl2��������Ȼ�����Һ�ò����ƣ���C����
D������������ɫͿ�ϵijɷ֣�ijЩ���������������Ϊ���ϣ���D��ȷ��
�ʴ�Ϊ��C��
���� ������Ҫ��������Һ���ƣ�����ϴ�ӣ�������ȵ�ʵ�������������Ԫ�ص����������IJⶨ��ͬʱ������ʵ��֪ʶ���ѶȲ���

| A�� | C5H10 | B�� | C7H8O | C�� | CH4O | D�� | C3H7Cl |
| A�� | ��ʯ�����ڹ�����ԭ�� | |
| B�� | ��ʯ���к���һ������ʯӢ���� | |
| C�� | ��ʯ�������������ǽ������� | |
| D�� | 1 mol��ʯ����ʹ1 molϡ���ᱻ��ԭ |
| A�� | ��ϩ�Ľṹ��ʽ��CH2CH2 | |
| B�� | ��������ģ��Ϊ | |
| C�� | �Ҵ����еĹ�������-OH����һ����λ����� | |
| D�� | ����ķ���ʽ��C2H4O |
| A�� | ��������Һ | B�� | ������Һ | C�� | Fe��OH��3���� | D�� | ��������Һ |
| A�� | 2��2-�������� | B�� | 1��2-���ȶ��� | C�� | 2-��-2-��ϩ | D�� | 2-��-3-��Ȳ |
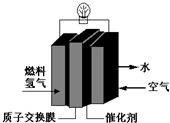 ��29����˻��ڼ䣬��Ϊ���������ܳ��͵������㳵��������װ�š���ɫ���ࡱ--���ӽ���Ĥȼ�ϵ�أ��乤��ԭ����ͼ��ʾ��
��29����˻��ڼ䣬��Ϊ���������ܳ��͵������㳵��������װ�š���ɫ���ࡱ--���ӽ���Ĥȼ�ϵ�أ��乤��ԭ����ͼ��ʾ��