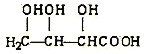��Ŀ����
����Ŀ������P(s)��Cl2(g)������Ӧ����PCl3(g)��PCl5(g)����Ӧ���̺�������ϵ��ͼ��ʾ(ͼ�е���H��ʾ����1mol���������)��
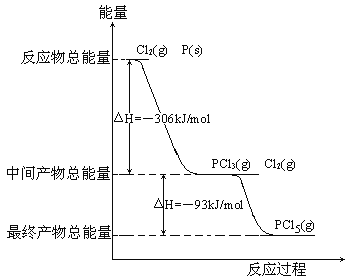
������ͼ�ش��������⣺
(1)P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽ_________________________________��
(2)PCl5�ֽ��PCl3��Cl2���Ȼ�ѧ����ʽ_________________________________�������ֽⷴӦ��һ�����淴Ӧ���¶�T1ʱ�����ܱ������м���0.80molPCl5����Ӧ�ﵽƽ��ʱPCl5��ʣ0.60mol����ֽ�����1����_________������Ӧ�¶���T1���ߵ�T2��ƽ��ʱPCl5�ķֽ���Ϊ��2����2_______��1(��������������С��������������)��
(3)��ҵ���Ʊ�PCl5ͨ�����������У��Ƚ�P��Cl2��Ӧ�����м����PCl3��Ȼ���£��ٺ�Cl2��Ӧ����PCl5��ԭ����________________________________________��
(4)P��Cl2��������Ӧ����1molPCl5����H3��_________��P��Cl2һ����Ӧ����1molPCl5����H4______��H3(��������������С��������������)��
(5)PCl5������ˮ��ַ�Ӧ���������������ᣬ�仯ѧ����ʽ��______________________________��
���𰸡���1��P(s)��Cl2(g)===PCl3(g)����H����306kJ��mol��1��
(2��PCl5(g)===PCl3(g)��Cl2(g)����H��93kJ��mol��1��25�������ڡ�
(3��������Ӧ��Ϊ���ȷ�Ӧ�������¶���������߲��ʣ���ֹ����ֽ⡣
(4����399kJ��mol��1�����ڡ�
(5��PCl5��4H2O===H3PO4��5HCl��
��������
��1����ͼ���Կ�����1molP��Cl2����ȫȼ�շų�������Ϊ306kJ��mol��1������P��Cl2��Ӧ����PCl3���Ȼ�ѧ��Ӧ����ʽΪP(s)��Cl2(g)===PCl3(g)����H����306kJ��mol��1��
(2���м����PCl3��δ��ȫ��Ӧ��Cl2���������������ղ���PCl5������������H����93kJ��mol��1������PCl5(g)===PCl3(g)��Cl2(g)����H��93kJ��mol��1���ֽ�����1����100����25�������Ȼ�ѧ��Ӧ����ʽ��֪���˷�Ӧ������ӦΪ���ȷ�Ӧ�����������¶ȣ�ƽ��������Ӧ�����ƶ���PCl5�ķֽ�����������2����1��
(3����ͼ��֪��P��Cl2��Ӧ����PCl3��PCl3��Cl2��һ����Ӧ����PCl5�����Ƿ��ȷ�Ӧ�������������ҵڶ��������¶ȣ�������PCl5�����ɣ���ֹPCl5�ķֽ⡣
(4���ɸ�˹���ɿ�֪��һ����������PCl5����������PCl5������ЧӦ��ȣ�����H3����399kJ��mol��1��
(5��PCl5��ˮ��Ӧ����H3PO4��HCl����ѧ����ʽΪ��PCl5��4H2O===H3PO4��5HCl��

 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�����Ŀ���л���X������ʽΪC4H6O5���㷺����������ˮ���У�����ƻ�������ѡ����ϡ�ɽ���Ϊ�ࡣ���ⶨ���л�������������ʣ����±������������ĿҪ����գ�
X������ | |
�� | X�������Ľ����Ʒ�Ӧ�������� |
�� | X�봼��������Ũ������������¾�����������ζ�IJ��� |
�� | ��һ��������X�ķ�������ˮ������ǻ�״�����������ˮ�����ӳɷ�Ӧ |
�� | 33.5gX��100mL��5mol/LNaOH��Һǡ����ȫ�к� |
��1��X�Ŀ��ܽṹ��ʽI��________����__________����__________��
��2����һ���������л���X�ɷ�����ѧ��Ӧ�������У�����ţ�__________��
A.ˮ�ⷴӦ B.ȡ����Ӧ C.�ӳɷ�Ӧ D.��ȥ��Ӧ E.�Ӿ۷�Ӧ F.�кͷ�Ӧ
��3������������X��Ϊͬϵ����ǣ�����ţ�_______����X��Ϊͬ���칹����ǣ�����ţ�_______��
(a)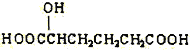 (b)
(b)![]()
(c)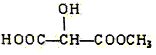 (d)H3COOC-COOCH3
(d)H3COOC-COOCH3
(e)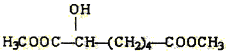 (f)
(f)