��Ŀ����
A��Z��R��D��E��F��G������ԭ���������������Ҳ�����20��Ԫ�أ����������ַǽ���Ԫ��λ��ͬһ���塣��֪A��Z��D������R�γ�ԭ�ӣ������ӣ�������ͬ�Ķ��ֳ����������ش������й����⡣
��1����X��Y��D��F�γɵ�����������Ӧ��ˮ���Ũ�Ⱦ�Ϊ0.1mol/L��X��Y��Һ��pH֮��Ϊ14����X��Y�ľ����۵���Ըߵ�Ϊ��д��ѧʽ�� ���������ֲ�����ڵ�ԭ���� ����ͬ�¶��£�������ʵ���Ũ�ȵ�X��Y���Ե���Һ�У���ˮ�������c��H+������Դ�С��ϵΪ ��
��2��A��Z��E�����γ��������幹�͵���̬����Q��P����֪P��ȼ����Ϊ1430kJ/mol������ȼ�յ��Ȼ�ѧ����ʽ ��
��3��R��D��F�䣻R��F��G������γɶ��ֻ����������������Ŀǰ�㷺ʹ�õľ�������ɱ�������Ļ��������ͬ���ʵ���Ũ�ȵ����������ʵ���Һ������ɱ��������ǿ�����ʵĻ�ѧʽΪ ����һ�����ʵ���Һ�У�������Ũ���ɴ�С��˳��Ϊ ��
��4����������Ԫ�ؼ����γɶ��ֳ�����Ԫ���������Z��G�γɵ�һ�ֻ�����������ѧ������������HC1��Һ�Ļ�ѧ��Ӧ����ʽΪ ��ZR2�ĵ���ʽ�� ���÷��ӵĿռ乹��Ϊ ������ZRF2�ĽṹʽΪ____
��5��R���γɶ��ֵ��ʣ���R2��R3���о��������½��Ƶõ�һ�����ΪR4�ķ����У�ÿ��Rԭ�Ӿ�����������Rԭ�Ӹ��γ�һ��������1molR4�й��õ��Ӷ���Ϊ____NA�����й�R4��˵������ȷ����
��R4��R3��R2��Ϊͬλ��
��R4������A��Z��D�ĵ��ʷ�Ӧ
��R4�и�ԭ��������Ϊ8���ӽṹ
��R4��1�����ͻ�����
��1����X��Y��D��F�γɵ�����������Ӧ��ˮ���Ũ�Ⱦ�Ϊ0.1mol/L��X��Y��Һ��pH֮��Ϊ14����X��Y�ľ����۵���Ըߵ�Ϊ��д��ѧʽ�� ���������ֲ�����ڵ�ԭ���� ����ͬ�¶��£�������ʵ���Ũ�ȵ�X��Y���Ե���Һ�У���ˮ�������c��H+������Դ�С��ϵΪ ��
��2��A��Z��E�����γ��������幹�͵���̬����Q��P����֪P��ȼ����Ϊ1430kJ/mol������ȼ�յ��Ȼ�ѧ����ʽ ��
��3��R��D��F�䣻R��F��G������γɶ��ֻ����������������Ŀǰ�㷺ʹ�õľ�������ɱ�������Ļ��������ͬ���ʵ���Ũ�ȵ����������ʵ���Һ������ɱ��������ǿ�����ʵĻ�ѧʽΪ ����һ�����ʵ���Һ�У�������Ũ���ɴ�С��˳��Ϊ ��
��4����������Ԫ�ؼ����γɶ��ֳ�����Ԫ���������Z��G�γɵ�һ�ֻ�����������ѧ������������HC1��Һ�Ļ�ѧ��Ӧ����ʽΪ ��ZR2�ĵ���ʽ�� ���÷��ӵĿռ乹��Ϊ ������ZRF2�ĽṹʽΪ____
��5��R���γɶ��ֵ��ʣ���R2��R3���о��������½��Ƶõ�һ�����ΪR4�ķ����У�ÿ��Rԭ�Ӿ�����������Rԭ�Ӹ��γ�һ��������1molR4�й��õ��Ӷ���Ϊ____NA�����й�R4��˵������ȷ����
��R4��R3��R2��Ϊͬλ��
��R4������A��Z��D�ĵ��ʷ�Ӧ
��R4�и�ԭ��������Ϊ8���ӽṹ
��R4��1�����ͻ�����

��

��ϰ��ϵ�д�
 ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д�
ѧ�ڸ�ϰһ��ͨѧϰ�ܶ�Ա��ĩ������ӱ����������ϵ�д� â���̸����������������ϵ�д�
â���̸����������������ϵ�д�
�����Ŀ
 �������ϱ�����ʵ�߲�ȫԪ�����ڱ��߽硣
�������ϱ�����ʵ�߲�ȫԪ�����ڱ��߽硣
 C2Ex����д����طŵ�ʱ�������ĵ缫��Ӧʽ��������Ӧʽ��_______________________���øõ������Դ���е�⺬��0.2 mol CuSO4��0.2 mol NaCl�Ļ����Һ500 mLʱ�����˵�ع���һ��ʱ�������23 g C���ʡ������������������Ϊ��________L����״���£���������Һ��ˮϡ����2 L ����Һ��pHΪ��________��
C2Ex����д����طŵ�ʱ�������ĵ缫��Ӧʽ��������Ӧʽ��_______________________���øõ������Դ���е�⺬��0.2 mol CuSO4��0.2 mol NaCl�Ļ����Һ500 mLʱ�����˵�ع���һ��ʱ�������23 g C���ʡ������������������Ϊ��________L����״���£���������Һ��ˮϡ����2 L ����Һ��pHΪ��________��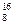 O��
O��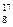 O��
O��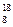 O��Ϊͬλ�أ���������Ԫ�أ���������ͬ
O��Ϊͬλ�أ���������Ԫ�أ���������ͬ