��Ŀ����
A��B��C��D��EΪ������̬��������A��B��C��ʹ����KMnO4��Һ��ɫ��1 molC����2mol�嵥����ȫ�ӳɣ��������ʷ�����ÿ��̼ԭ���϶���һ����ԭ�ӡ�A��C������ͬ��ͨʽ��A��H2�ӳɵ�B��B����ͬ������N2�ܶ���ͬ��D�����е����к�̼����������͵ģ�E��D��ͬϵ���ȫȼ�յ����ʵ���B��E����CO2������ͬ����ش�
��1��д��C�Ľṹ��ʽ����������������������
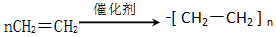
��2��B�ĵ���ʽ�������������������γɸ߷��ӻ�����Ļ�ѧ����ʽ����������������
��3����̼ԭ����4��n��10ʱ����D��E��ͬϵ���У���һ�Ȼ���û��ͬ���칹�壬���ȴ�����3��ͬ���칹�������������������������������������д���ƣ���
��4����A��B��C��D�����ʵ�����ϣ�120��ʱ��ȡVL�������6VLO2�г��ȼ�գ������¶Ȳ��䣬��Ӧ�����������Ϊ����������������������
��8�֣�
��1��CH2=CH��CH=CH2
��2����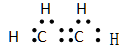
��3��2,2,3,3���ļ�����
��4��7VL
���������������1��BΪ��̬���� B����ͬ������N2�ܶ���ͬ��B�ķ�������28����B��C2H4��A��H2�ӳɵ�B��A��C2H2��A��C������ͬ��ͨʽ��1 molC����2mol�嵥����ȫ�ӳɣ��������ʷ�����ÿ��̼ԭ���϶���һ����ԭ�ӣ�C���и�̼̼˫������CΪC4H6���ṹ��ʽΪCH2=CH��CH=CH2��
��2��B��C2H4������ʽΪ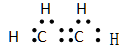 ��B�����Ӿ۷�Ӧ������ʽΪ
��B�����Ӿ۷�Ӧ������ʽΪ ��
��
��3��D�����е����к�̼����������͵ģ�E��D��ͬϵ���ȫȼ�յ����ʵ���B��E����CO2������ͬ��DΪ���飬��C4H10, ��D��E��ͬϵ���У���һ�Ȼ���û��ͬ���칹�壬���ȴ�����3��ͬ���칹����2,2,3,3���ļ����顣
��4��120��ʱ����Ӧ��������ﶼ����̬�����������غ㶨�ɵģ���Ӧ�����������Ϊ7VL��
���㣺�л��ƶ� �л�����
���������⿼������л��ƶϺ��л���������֪ʶ����Ŀ�Ѷȴ���ѧ���Ի���֪ʶ�����ա�Ӧ�úͽ�����������

��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⣮
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ���
�Σ�
��3������Cԭ�ӵĵ����Ų�ͼ
��4����֪BA5Ϊ���ӻ����д�������ʽ
��5��DE3����ԭ�ӵ��ӻ���ʽΪ
��6���õ���ʽ��ʾFԪ����EԪ���γɻ�������γɹ���
 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�

