��Ŀ����
ijС��ͬѧ����ͼ��ʾװ������Ũ�����ľ̿��Ӧ��ʵ�飺C+2H2SO4��Ũ��
| ||
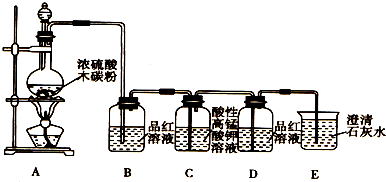
�ش��������⣺
��1��װ��B�й۲쵽��������
��2��װ��C������KMnO4��Һ���ڳ�ȥSO2����������SO2����
�ף���ֹSO2����CO2�ļ��� �ң�������CO2����
��3��װ��D��Ʒ����Һ����ɫ����˵��SO2
��4��װ��E�й۲쵽��������
��5������Aװ�����ɵĻ������ֱ��ͨ�뵽ʢ��Ba��NO3��2��Һ���ձ��У����ձ��в����ij�����
������̼��Ũ�����ڼ��������·�Ӧ����SO2��CO2��װ��B��Ʒ����Һ���ڼ������SO2��SO2��CO2����ʹ����ʯ��ˮ����ǣ�������ó���ʯ��ˮ����CO2֮ǰ�����SO2���ոɾ���װ��C���Ը��������Һ��������SO2��װ��D��Ʒ����Һ���ڼ���SO2�Ƿ���ɾ���װ��E����ʯ��ˮ���ڼ������CO2��
��1��SO2����Ư���ԣ�����ʹƷ����Һ��ɫ����˳���Ʒ����Һ����SO2��
��2�����Ը�����ؾ���ǿ�����ԣ�����������������SO2��CO2����ʹ����ʯ��ˮ����ǣ�������ó���ʯ��ˮ����CO2֮ǰ�����SO2���ոɾ���
��3��װ��D��Ʒ����Һ����ɫ��˵��SO2���ɾ���
��4��װ��E�г����ʯ��ˮ����ǣ�˵��������һ������CO2���壬CO2���������Ʒ�Ӧ����̼��Ƴ�����
��5��̼�ᱵ�������ᣬ��˲�����̼�ᱵ���ɣ���������������������Ϊ��������ӣ�����������뱵���ӷ�Ӧ���ɲ�����������ᱵ������
��1��SO2����Ư���ԣ�����ʹƷ����Һ��ɫ����˳���Ʒ����Һ����SO2��
��2�����Ը�����ؾ���ǿ�����ԣ�����������������SO2��CO2����ʹ����ʯ��ˮ����ǣ�������ó���ʯ��ˮ����CO2֮ǰ�����SO2���ոɾ���
��3��װ��D��Ʒ����Һ����ɫ��˵��SO2���ɾ���
��4��װ��E�г����ʯ��ˮ����ǣ�˵��������һ������CO2���壬CO2���������Ʒ�Ӧ����̼��Ƴ�����
��5��̼�ᱵ�������ᣬ��˲�����̼�ᱵ���ɣ���������������������Ϊ��������ӣ�����������뱵���ӷ�Ӧ���ɲ�����������ᱵ������
����⣺��1��SO2����Ư���ԣ�����ʹƷ����Һ��ɫ������Ʒ����Һ����SO2�����װ��B��Ʒ����Һ���ڼ������SO2��
�ʴ�Ϊ��Ʒ����ɫ��SO2��Ư���ԣ�
��2�����Ը�����ؾ���ǿ�����ԣ�������������������������Ը��������Һ����SO2�������˶�������Ļ�ԭ�ԣ�SO2��CO2����ʹ����ʯ��ˮ����ǣ�������ó���ʯ��ˮ����CO2֮ǰ�����SO2���ոɾ���
�ʴ�Ϊ����ԭ�ԣ��ף�
��3��SO2����Ư���ԣ�����ʹƷ����Һ��ɫ��װ��D��Ʒ����Һ����ɫ��˵��SO2���ɾ���
�ʴ�Ϊ���ѳ�����
��4��װ��E�г����ʯ��ˮ����ǣ�˵��������һ������CO2���壬CO2���������Ʒ�Ӧ����̼��Ƴ�������ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
�ʴ�Ϊ������ʯ��ˮ����ǣ� CO2��CO2+Ca��OH��2=CaCO3��+H2O��
��5��SO2��CO2����ˮ�������������̼�ᣬ̼�ᱵ�������ᣬ��˲�����̼�ᱵ���ɣ���������������������Ϊ��������ӣ�����������뱵���ӷ�Ӧ���ɲ�����������ᱵ������
�ʴ�Ϊ��BaSO4��
�ʴ�Ϊ��Ʒ����ɫ��SO2��Ư���ԣ�
��2�����Ը�����ؾ���ǿ�����ԣ�������������������������Ը��������Һ����SO2�������˶�������Ļ�ԭ�ԣ�SO2��CO2����ʹ����ʯ��ˮ����ǣ�������ó���ʯ��ˮ����CO2֮ǰ�����SO2���ոɾ���
�ʴ�Ϊ����ԭ�ԣ��ף�
��3��SO2����Ư���ԣ�����ʹƷ����Һ��ɫ��װ��D��Ʒ����Һ����ɫ��˵��SO2���ɾ���
�ʴ�Ϊ���ѳ�����
��4��װ��E�г����ʯ��ˮ����ǣ�˵��������һ������CO2���壬CO2���������Ʒ�Ӧ����̼��Ƴ�������ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O��
�ʴ�Ϊ������ʯ��ˮ����ǣ� CO2��CO2+Ca��OH��2=CaCO3��+H2O��
��5��SO2��CO2����ˮ�������������̼�ᣬ̼�ᱵ�������ᣬ��˲�����̼�ᱵ���ɣ���������������������Ϊ��������ӣ�����������뱵���ӷ�Ӧ���ɲ�����������ᱵ������
�ʴ�Ϊ��BaSO4��
���������⿼����Ũ�����ǿ�����ԣ�������SO2��CO2���ʵıȽϣ�SO2��CO2����ʹ����ʯ��ˮ����ǣ�������ó���ʯ��ˮ����CO2֮ǰ�����SO2���ոɾ�����5��ע�������ǿ�����ԣ�Ϊ��Ƶ���㣬����������ѧ�������õĿ�ѧ���������ѧ��ѧϰʵ��Ļ����ԣ��Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
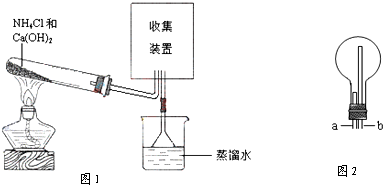
ijУ����ʵ��С��ͬѧ���ͼ6-5��ʾװ�ý���ʵ��(�г�װ������ȥ)��

ͼ6-5
(1)��С��ͬѧ����ͼ��ʾװ�ý��С���Ȳ����ȡ��ȼ��������֤��ʵ�顣
����ȡ��Ȳ�Ļ�ѧ����ʽ��________________________________________��
�ڵ�ȼ��Ȳǰ����Ҫ____________________��
���ڵ��ܿ�c����ȼ��Ȳ���۲쵽��������____________________��
(2)��С��ͬѧ����ͼװ�ý���ʵ�飬ȷ��ij���ʹ��Ľṹ��
�ٷ�Ӧǰ���ȶ������ܽ��е�һ�ζ�������Ӧ��װ���¶���ȴ�����£��ٶ������ܽ��еڶ��ζ���������ʱ��Ӧע��IJ�����______________________________����ʹ�����밼Һ����ʹ���ƽ��
��ʵ�����ݼ�¼���£�(���ж������ۺϳɱ�״���µ���ֵ)
| ���ʹ������� | �����Ƶ����� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
�� | 40 mL | 264 mL | ||
�� | 40 mL | 152 mL |
��֪�ñ��ʹ�����Է�������Ϊ62�������������ݿ�ȷ���ñ��ʹ���________Ԫ����
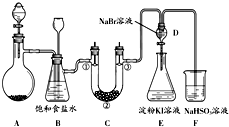 ij����С��ͬѧ����ͼ��ʾװ����ʵ�����Ʊ�������̽����������ʣ��г��豸���ԣ���
ij����С��ͬѧ����ͼ��ʾװ����ʵ�����Ʊ�������̽����������ʣ��г��豸���ԣ���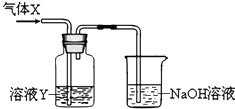
 ij��ȤС��ͬѧ����ͼ��ʾװ����ȡ������̽�����������ʣ�
ij��ȤС��ͬѧ����ͼ��ʾװ����ȡ������̽�����������ʣ�