��Ŀ����
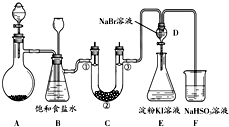
 ij����С��ͬѧ����ͼ��ʾװ����ʵ�����Ʊ�������̽����������ʣ��г��豸���ԣ���
ij����С��ͬѧ����ͼ��ʾװ����ʵ�����Ʊ�������̽����������ʣ��г��豸���ԣ�����1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��ص����ӷ�Ӧ����ʽΪ��
��2��װ��B������֮һ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т١��ڡ������η���
| �� | �� | �� | |
| a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
| b | �������ɫ���� | �� �� | ʪ�����ɫ���� |
| c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
| d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
��5����ͬѧ�����ʵ�鷽�����в��㣬��˵���������㲻�㼰ԭ���ǣ���
��������1������Ư���ijɷ�Ϊ�������ƾ��������ԣ��������������е������ӣ�����������ԭ��Ӧ�����غ�͵���غ㡢ԭ���غ�д����
��2������װ��������ѹǿ�仯�ͳ���©���� Һ��Һ��仯�����жϣ�
��3������������Ư�����ã�������ˮ��Ӧ���ɴ��������Ư���Խ���������ƣ�
��4�������������廯�Ʒ�Ӧ�����嵥�ʣ��嵥��ˮ��Һ��Ϊ��ɫ���ɫ��Һ���嵥�ʺ͵⻯����Һ�еĵ⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ���������۱���ɫ��
��5������װ��ͼ�������������Eװ���� D�������ų������ĸ��ţ�װ��F�������������ɵ���Ⱦ�������������
��2������װ��������ѹǿ�仯�ͳ���©���� Һ��Һ��仯�����жϣ�
��3������������Ư�����ã�������ˮ��Ӧ���ɴ��������Ư���Խ���������ƣ�
��4�������������廯�Ʒ�Ӧ�����嵥�ʣ��嵥��ˮ��Һ��Ϊ��ɫ���ɫ��Һ���嵥�ʺ͵⻯����Һ�еĵ⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ���������۱���ɫ��
��5������װ��ͼ�������������Eװ���� D�������ų������ĸ��ţ�װ��F�������������ɵ���Ⱦ�������������
����⣺��1��Ư���ijɷ�Ϊ�������ƾ��������ԣ��������������е������ӣ���ӦΪ����������Ӻ�������������Һ�з���������ԭ��Ӧ����Ӧ�����ӷ���ʽΪ��ClO-+Cl-+2H+=Cl2��+H2O��
�ʴ�Ϊ��ClO-+Cl-+2H+=Cl2��+H2O��
��2��װ��B������֮һ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������װ��AB������ѹǿ�����B�е�Һ��ѹ�볤��©�����γ�ˮ����
�ʴ�Ϊ�������г���©����Һ�������������γ�ˮ����
��3��������Ư�����ã�������ˮ��Ӧ���ɴ��������Ư���ԣ�װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�
a��������ʳ��ˮ�г����Ậ��ˮ������������ɫ��������ɫ��ͨ����ʯ�Һ������ᱻ���գ������������������Ƿ����Ư���ԣ���a����
b��������ʳ��ˮ�г����Ậ��ˮ������������ɫ��������ɫ��ͨ���轺��ˮ�����ᱻ���գ�������ʪ�����ɫ��������ɫ������֤����Ư�����õ����ʣ���b����
c��������ʳ��ˮ�г����Ậ��ˮ������ʪ����ɫ��������ɫ������Ũ���ᣬ���岻�ܽ������װ�ã�����֤�������Ƿ���Ư���ԣ���c����
d��������ʳ��ˮ�г����Ậ��ˮ������ʪ����ɫ��������ɫ��ͨ�����������Ȼ�������ˮ�����������ɫ��������ɫ֤��������Ư���ԣ�����C��I��II��III���η���ʪ�����ɫ��������ˮ�Ȼ��ơ��������ɫ����������ѡd��
�ʴ�Ϊ��d��
��4��D�����廯�ƣ�����D�л���ͨ����������ʱ���������廯�Ʒ�Ӧ�����嵥�ʣ����Կ�����ɫ��Һ��Ϊ�ƣ���������Ϊ����Һ����ɫ�仯Ϊ��ɫ��������ԭ��Ӧ���������������Դ����������˵��������������ǿ���壻��������װ��D�к��嵥�ʵ�������Һ���뺬�⻯�ص�����Һ��װ��E�У��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�������۱�����
�ʴ�Ϊ���ƣ���ȣ��� ��Һ����ɫ��
��5��װ��������֤�嵥�������Դ��ڵⵥ�ʵ�ʵ����֤������ �����ų��������������ӵĸ������ã����װ��F����ͨ����ˮ��Ӧ�������ᣬ��������������Ʒ�Ӧ���ɶ���������Ⱦ�������ܱ����գ�
�ʴ�Ϊ����E��δ���ų�D�й���Cl2�ĸ��ţ����û���I2��
��Cl2ͨ��NaHSO3��Һ�л����SO2����Ⱦ������
�ʴ�Ϊ��ClO-+Cl-+2H+=Cl2��+H2O��
��2��װ��B������֮һ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������װ��AB������ѹǿ�����B�е�Һ��ѹ�볤��©�����γ�ˮ����
�ʴ�Ϊ�������г���©����Һ�������������γ�ˮ����
��3��������Ư�����ã�������ˮ��Ӧ���ɴ��������Ư���ԣ�װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�
a��������ʳ��ˮ�г����Ậ��ˮ������������ɫ��������ɫ��ͨ����ʯ�Һ������ᱻ���գ������������������Ƿ����Ư���ԣ���a����
b��������ʳ��ˮ�г����Ậ��ˮ������������ɫ��������ɫ��ͨ���轺��ˮ�����ᱻ���գ�������ʪ�����ɫ��������ɫ������֤����Ư�����õ����ʣ���b����
c��������ʳ��ˮ�г����Ậ��ˮ������ʪ����ɫ��������ɫ������Ũ���ᣬ���岻�ܽ������װ�ã�����֤�������Ƿ���Ư���ԣ���c����
d��������ʳ��ˮ�г����Ậ��ˮ������ʪ����ɫ��������ɫ��ͨ�����������Ȼ�������ˮ�����������ɫ��������ɫ֤��������Ư���ԣ�����C��I��II��III���η���ʪ�����ɫ��������ˮ�Ȼ��ơ��������ɫ����������ѡd��
�ʴ�Ϊ��d��
��4��D�����廯�ƣ�����D�л���ͨ����������ʱ���������廯�Ʒ�Ӧ�����嵥�ʣ����Կ�����ɫ��Һ��Ϊ�ƣ���������Ϊ����Һ����ɫ�仯Ϊ��ɫ��������ԭ��Ӧ���������������Դ����������˵��������������ǿ���壻��������װ��D�к��嵥�ʵ�������Һ���뺬�⻯�ص�����Һ��װ��E�У��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�������۱�����
�ʴ�Ϊ���ƣ���ȣ��� ��Һ����ɫ��
��5��װ��������֤�嵥�������Դ��ڵⵥ�ʵ�ʵ����֤������ �����ų��������������ӵĸ������ã����װ��F����ͨ����ˮ��Ӧ�������ᣬ��������������Ʒ�Ӧ���ɶ���������Ⱦ�������ܱ����գ�
�ʴ�Ϊ����E��δ���ų�D�й���Cl2�ĸ��ţ����û���I2��
��Cl2ͨ��NaHSO3��Һ�л����SO2����Ⱦ������
���������⿼��������ʵ������ȡ��������ѧ���ʵ�Ӧ�ã�ʵ����ƣ�ʵ��װ�õ�ԭ����������ѧ����ʽ����д����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
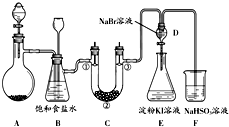
 ij����С��ͬѧ����ͼ��ʾװ����ʵ�����Ʊ�������̽����������ʣ��г��豸���ԣ���
ij����С��ͬѧ����ͼ��ʾװ����ʵ�����Ʊ�������̽����������ʣ��г��豸���ԣ���
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��ص����ӷ�Ӧ����ʽΪ��
______��
��2��װ��B������֮һ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����______��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т١��ڡ������η���______����ѡ����ĸ��
| �� | �� | �� | |
| a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
| b | �������ɫ���� | �衡 �� | ʪ�����ɫ���� |
| c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
| d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
��5����ͬѧ�����ʵ�鷽�����в��㣬��˵���������㲻�㼰ԭ���ǣ���______����______��
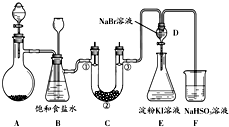
ij����С��ͬѧ����ͼ��ʾװ����ʵ�����Ʊ�������̽����������ʣ��г��豸���ԣ���
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��ص����ӷ�Ӧ����ʽΪ��
��
��2��װ��B������֮һ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т١��ڡ������η��� ����ѡ����ĸ��
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԣ�����D�л���ͨ��һ��������ʱ�����Կ�����ɫ��Һ��Ϊ ɫ��˵���ȵķǽ����Դ����壻֮���������װ��D��������Һ����װ��E�У����۲쵽�������� ����˵����ķǽ����Դ��ڵ⣻
��5����ͬѧ�����ʵ�鷽�����в��㣬��˵���������㲻�㼰ԭ���ǣ��� ���� ��
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��ص����ӷ�Ӧ����ʽΪ��
��
��2��װ��B������֮һ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т١��ڡ������η��� ����ѡ����ĸ��
| �� | �� | �� | |
| a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
| b | �������ɫ���� | �� �� | ʪ�����ɫ���� |
| c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
| d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
��5����ͬѧ�����ʵ�鷽�����в��㣬��˵���������㲻�㼰ԭ���ǣ��� ���� ��

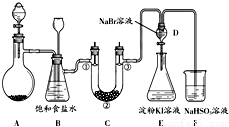
ij����С��ͬѧ����ͼ��ʾװ����ʵ�����Ʊ�������̽����������ʣ��г��豸���ԣ���
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��ص����ӷ�Ӧ����ʽΪ��
��
��2��װ��B������֮һ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т١��ڡ������η��� ����ѡ����ĸ��
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԣ�����D�л���ͨ��һ��������ʱ�����Կ�����ɫ��Һ��Ϊ ɫ��˵���ȵķǽ����Դ����壻֮���������װ��D��������Һ����װ��E�У����۲쵽�������� ����˵����ķǽ����Դ��ڵ⣻
��5����ͬѧ�����ʵ�鷽�����в��㣬��˵���������㲻�㼰ԭ���ǣ��� ���� ��
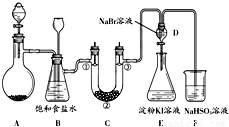
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾������Ũ���ᣬ��ص����ӷ�Ӧ����ʽΪ��
��
��2��װ��B������֮һ�ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т١��ڡ������η��� ����ѡ����ĸ��
| �� | �� | �� | |
| a | �������ɫ���� | ��ʯ�� | ʪ�����ɫ���� |
| b | �������ɫ���� | �� �� | ʪ�����ɫ���� |
| c | ʪ�����ɫ���� | Ũ���� | �������ɫ���� |
| d | ʪ�����ɫ���� | ��ˮ�Ȼ��� | �������ɫ���� |
��5����ͬѧ�����ʵ�鷽�����в��㣬��˵���������㲻�㼰ԭ���ǣ��� ���� ��