��Ŀ����
����Ŀ������������Ӧ��ʵ������������£�
A�����Թ�����ע������NaOH��Һ�����������֮���NaOH��Һ��ȥ����������ˮϴ���Թܱ���
B����ϴ�����Թ�������������Һ
C����������Һ�е���3��4����ȩϡ��Һ
D������
��ش��������⣺
��1������A�м�NaOH��Һ��������е�Ŀ����_____________________________;
��2������������Һ�����ƹ��̣�__________________________________________;
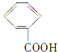
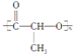
��3��д����ȩ����������Ӧ�Ļ�ѧ����ʽ��_____________________________________;
��4��������Һ���ÿ��ܻᱬը��ֱ���ŷŻ���Ⱦ�������������Դ�˷ѣ�ʵ���Ҵӷ�Һ�л�������ʵ���������£�![]()
��֪��[Ag(NH3)2]��![]() Ag����2NH3
Ag����2NH3
��д����Һ��ϡ���ᷴӦ�����ӷ���ʽ��__________________________________;
�ڼ�������Ҫ������Ŀ����___________________________________________��
�۸�ʵ������п��ܲ����Ĵ�����Ⱦ����________________________________��
���𰸡� ��ȥ�Թ��ڱڵ����� ��ྻ���Թ��м���2%��������Һ��Ȼ������Թܱ���ε���2%��ϡ��ˮ������������ij���ǡ���ܽ�Ϊֹ CH3CHO��2Ag(NH3)2OH![]() CH3COONH4��2Ag����3NH3��H2O [Ag(NH3)2]����OH����3H��=Ag����2NH4����H2O �������ӻ�ԭ NO
CH3COONH4��2Ag����3NH3��H2O [Ag(NH3)2]����OH����3H��=Ag����2NH4����H2O �������ӻ�ԭ NO
��������������Ӧ�ɹ��Ĺؼ�֮һ���Թܱ���ྻ���Թ����ȼ�������������������Һ����������У�Ŀ�ľ��dz�ȥ�Թ��ڱ��ϵ����ۣ���֤�ԹܵĽྻ��������Һ������һ��Ҫ�ȼ�����������Ȼ������Թܱ���ε���ϡ��ˮ������������ij���ǡ���ܽ�Ϊֹ��������Һ�ܹ���ȩ������Ϊ�Ȼ�����������ԭΪ����������ϡ����ʱ��������Һ���������ӷ�Ӧ���ӷ�Һ�л���������������Ϊ�˽��������û�Ϊ���������ﵽ���յ�Ŀ�ģ�ͬʱ������ؽ������ӵ���Ⱦ�������Ϸ������
��1��������Ӧ�ɹ��Ĺؼ�֮һ���Թܱ���ྻ���Թ����ȼ�������������������Һ����������У�Ŀ�ľ��dz�ȥ�Թ��ڱ��ϵ����ۣ���֤�ԹܵĽྻ����ȷ������ȥ�Թ��ڱڵ�������
��2����ྻ���Թ��м���2%��������Һ��Ȼ������Թܱ���ε���2%��ϡ��ˮ������������ij���ǡ���ܽ�Ϊֹ��������ҺΪ������Һ����ȷ������ྻ���Թ��м���2%��������Һ��Ȼ������Թܱ���ε���2%��ϡ��ˮ������������ij���ǡ���ܽ�Ϊֹ��
��3��д����ȩ��������Һˮԡ���ȷ�Ӧ���������������������ˮ����Ӧ�Ļ�ѧ����ʽ��CH3CHO��2Ag(NH3)2OH![]() CH3COONH4��2Ag����3NH3��H2O ����ȷ����CH3CHO��2Ag(NH3)2OH
CH3COONH4��2Ag����3NH3��H2O ����ȷ����CH3CHO��2Ag(NH3)2OH![]() CH3COONH4��2Ag����3NH3��H2O��
CH3COONH4��2Ag����3NH3��H2O��
��4����������Һ�����ᷴӦ����������������狀�ˮ����Ӧ�����ӷ���ʽ��[Ag(NH3)2]����OH����3H��=Ag����2NH4����H2O ����ȷ����[Ag(NH3)2]����OH����3H��=Ag����2NH4����H2O ��
�ڼ�������Ҫ�������������ĵ�������ͬʱ�����û���������ȷ�𰸣��������ӻ�ԭ��
�������������ᷴӦ����һ�������ȶԻ�������Ⱦ����������ȷ����NO��

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�����Ŀ��ijʵ��С���о���Һ��AgNO3��Na2S�ķ�Ӧ��
ʵ�� | �Լ� | ���� | |
| �Թ� | �ι� | |
��pH = 4�� |
��pH = 9�� | ���ֺ�ɫ���� | |
��1�������ӷ���ʽ����Na2S��ҺpH > 7��ԭ��________��
��2��ʵ��С��ͬѧ��Ϊ��ɫ�����п��ܺ���Ag2O��Ag2S��Ag�����ʵ����֤��
��֪��i��Ũ�����ܽ�Ag2Sת��Ϊ![]() ��
��![]() ��
��
ii��Ag2O���ܽ���Ũ��ˮ���γ�������Һ����Ag2S��Ag�����ܡ�
�� ��Ʋ�ʵʩ����ʵ�飬֤ʵ�����к���Ag2S��

�Լ�1���Լ�2�ֱ���_________��_________��
����1������2�ֱ���_________��_________��
�� ��Ʋ�ʵʩ����ʵ�飬֤ʵ������������Ag2O����ʵ�����������������
ʵ����� | ʵ������ | |
����i | ȡ����������Һ�������еμ����� | ���ְ�ɫ���� |
����ii | ȡ����ϴ�Ӻ�ĺ�ɫ������____________ | ____________ |
�� �����飬����������Ag��

��3��ʵ��С��ͬѧ��ΪAgNO3��Һ���������ԣ���һ���������ܹ�����Na2S�����ʵ������о���ʵ��װ������ͼ��ʾ������õ�ѹΪa��![]() ����
����
��AgNO3��Һ������![]() �����ʽ����Ʋ⣺
�����ʽ����Ʋ⣺
����1�� ![]() ��AgNO3��Һ��
��AgNO3��Һ��![]() ������
������![]() ��
��
����2�� ![]() ��AgNO3��Һ��
��AgNO3��Һ��![]() ������
������![]() ��
��
������ͼװ�ü����о�����֪����ѹ��С��ӳ������������ԭ��ǿ���IJ��죻�����������뻹ԭ��ǿ������Խ��ѹԽ��
�� ��![]() ��AgNO3��Һ�滻Ϊ_______��Һ����¼��ѹΪb��
��AgNO3��Һ�滻Ϊ_______��Һ����¼��ѹΪb��![]() ����
����
�� ����ʵ��֤ʵ������![]() ��������һ������
��������һ������![]() ����֤����______��
����֤����______��
ʵ����ۣ�AgNO3��Һ��Na2S��Һ�ķ�Ӧ�����뷴Ӧ�����йء�