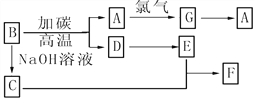
Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°Ņń≥ Ķ—ť–°◊ťĶńÕ¨—ß”√Ō¬Ń–◊į÷√ĹÝ––““īľīŖĽĮ—űĽĮĶń Ķ—ť£¨«ŽĽōīūŌ¬Ń–ő Ő‚£ļ
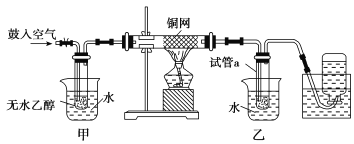
£®1£© Ķ—ťĻż≥Ő÷–Õ≠ÕÝ≥ŲŌ÷ļž…ęļÕļŕ…ęĹĽŐśĶńŌ÷Ōů£¨«Ž–ī≥ŲŌŗ”¶ĶńĽĮ—ß∑ī”¶∑Ĺ≥Ő Ĺ
__________________________________________°£
Ō®√ūĺ∆ĺęĶ∆£¨≤Ľ∂ŌĶōĻń»ŽŅ’∆Ý£¨∑ī”¶»‘ń‹ľŐ–ÝĹÝ––£¨ňĶ√ų““īľĶńīŖĽĮ—űĽĮ∑ī”¶ «_______∑ī”¶°£
£®2£©ľ◊ļÕ““ŃĹłŲňģ‘°◊ų”√≤ĽŌŗÕ¨£¨ľ◊Ķń◊ų”√ «_____________£Ľ““Ķń◊ų”√ «_____________°£
£®3£©∑ī”¶ĹÝ––“Ľ∂ő Īľšļů£¨ľĮ∆Ý∆Ņ÷– ’ľĮĶĹĶń∆ÝŐŚĶń÷ų“™≥…∑÷ «__________°£
£®4£©»Ű ‘Ļ‹a÷– ’ľĮĶĹĶń“ļŐŚ”√◊Ō…ę Į»Ô ‘÷Ĺľž—ť£¨ ‘÷ĹŌ‘ļž…ę£¨ňĶ√ų“ļŐŚ÷–ļ¨”–_______£¨“™≥ż»•ł√őÔ÷ £¨Ņ…Ō»‘༞ļŌ“ļ÷–ľ”»Ž________(ŐÓ◊÷ńł–ÚļŇ)£¨»Ľļů‘ŔÕ®Ļż________(ŐÓ≤Ŕ◊ų√Ż≥∆)ľīŅ…≥ż»•°£
a£ģ¬»ĽĮń∆»‹“ļ b£ģĪĹ c£ģŐľňŠ«‚ń∆»‹“ļ d£ģňń¬»ĽĮŐľ
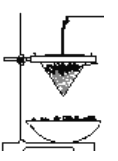
°ĺīūįł°Ņ 2Cu+O2![]() 2CuO CH3CH2OH+CuO
2CuO CH3CH2OH+CuO![]() CH3CHO+Cu+H2O ∑Ň»» ľ”»»““īľ£¨≤ķ…ķ““īľ’Ű∆Ý ľ”»»““īľ£¨Ī„”ŕ““īľĶńĽ”∑Ę£Ľņš»ī£¨Ī„”ŕ““»©Ķń ’ľĮ£Ľ Ķ™∆Ý£®N2£© ““ňŠ c ’ŰŃů
CH3CHO+Cu+H2O ∑Ň»» ľ”»»““īľ£¨≤ķ…ķ““īľ’Ű∆Ý ľ”»»““īľ£¨Ī„”ŕ““īľĶńĽ”∑Ę£Ľņš»ī£¨Ī„”ŕ““»©Ķń ’ľĮ£Ľ Ķ™∆Ý£®N2£© ““ňŠ c ’ŰŃů
°ĺĹ‚őŲ°Ņ(1)““īľĶńīŖĽĮ—űĽĮ∑ī”¶Ļż≥Ő£ļĹū ŰÕ≠ĪĽ—ű∆Ý—űĽĮő™—űĽĮÕ≠£¨2Cu+O2![]() 2CuO£¨—űĽĮÕ≠Ĺę““īľ—űĽĮő™““»©£¨CH3CH2OH+CuO
2CuO£¨—űĽĮÕ≠Ĺę““īľ—űĽĮő™““»©£¨CH3CH2OH+CuO![]() CH3CHO+Cu+H2O£¨∑ī”¶–Ť“™ľ”»»ĹÝ––£¨Õ£÷Ļľ”»»∑ī”¶»‘ľŐ–ÝĹÝ––£¨ňĶ√ų““īľĶń—űĽĮ∑ī”¶ «∑Ň»»∑ī”¶£ĽĻ īūįłő™£ļ2Cu+O2
CH3CHO+Cu+H2O£¨∑ī”¶–Ť“™ľ”»»ĹÝ––£¨Õ£÷Ļľ”»»∑ī”¶»‘ľŐ–ÝĹÝ––£¨ňĶ√ų““īľĶń—űĽĮ∑ī”¶ «∑Ň»»∑ī”¶£ĽĻ īūįłő™£ļ2Cu+O2![]() 2CuO°ĘCH3CH2OH+CuO
2CuO°ĘCH3CH2OH+CuO![]() CH3CHO+Cu+H2O£Ľ∑Ň»»£Ľ
CH3CHO+Cu+H2O£Ľ∑Ň»»£Ľ
(2)ľ◊ļÕ““ŃĹłŲňģ‘°◊ų”√≤ĽŌŗÕ¨£¨ľ◊ «»»ňģ‘°£¨◊ų”√ «““īľ∆Ĺő»∆ÝĽĮ≥…““īľ’Ű∆Ý£¨““ «ņšňģ‘°£¨ńŅĶń «Ĺę““»©ņš»īŌ¬ņī£¨Ļ īūįłő™£ļľ”»»““īľ£¨Ī„”ŕ““īľĶńĽ”∑Ę£Ľņš»ī£¨Ī„”ŕ““»©Ķń ’ľĮ£Ľ
(3)Ņ’∆Ý÷–—ű∆ÝĪĽ∑ī”¶Ńň£¨ľĮ∆Ý∆Ņ÷– ’ľĮĶń∆ÝŐŚ÷ų“™ «Ķ™∆Ý£¨Ļ īūįłő™£ļĶ™∆Ý£Ľ
(4)»Ű ‘Ļ‹a÷– ’ľĮĶĹĶń“ļŐŚ”√◊Ō…ę Į»Ô ‘÷Ĺľž—ť£¨ ‘÷ĹŌ‘ļž…ę£¨ňĶ√ų“ļŐŚ÷–ĽĻļ¨”–““ňŠ£¨ňńłŲ—°‘Ůīūįł÷–£¨÷Ľ”–ŐľňŠ«‚ń∆Ņ…“‘ļÕ““ňŠ∑ī”¶£¨…ķ≥…““ňŠń∆°ĘňģļÕ∂Ģ—űĽĮŐľ£¨ ĶŌ÷ŃĹ÷÷Ľ•»‹őÔ÷ Ķń∑÷ņŽ”√’ŰŃů∑®£¨Ļ īūįłő™£ļ““ňŠ£Ľc£Ľ’ŰŃů°£

°ĺŐ‚ńŅ°Ņ”√“ĽľŘņŽ◊”◊ť≥…ňń÷÷—ő£¨AC°ĘBD°ĘAD°ĘBCĶń1mol°§L-1 »‹“ļ£¨‘ŕ “ő¬Ō¬«įŃĹ÷÷»‹“ļĶńpH£Ĺ7£¨Ķ໿÷÷»‹“ļĶńpH >7£¨◊Óļů“Ľ÷÷»‹“ļĶńpH< 7£¨‘Ú°£
A | B | C | D | |
ľÓ–‘ | AOH>BOH | AOH<BOH | AOH>BOH | AOH<BOH |
ňŠ–‘ | HC>HD | HC>HD | HC<HD | HC<HD |
A. A B. B C. C D. D