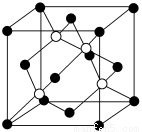
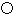ΧβΡΩΡΎ»ί
AlΘ≠Ag2OΒγ≥Ί «“Μ÷÷Ω…”ΟΉςΥ°œ¬Ε·ΝΠΒΡ”≈ΝΦΒγ‘¥Θ§Τδ‘≠άμ»γΆΦΥυ ΨΓΘΗΟΒγ≥ΊΙΛΉς ±ΉήΖ¥”Π ΫΈΣ2AlΘΪ3Ag2OΘΪ2NaOH=2NaAlO2ΘΪ6AgΘΪH2OΘ§‘ρœ¬Ν–ΥΒΖ®¥μΈσΒΡ « (ΓΓΓΓ)ΓΘ
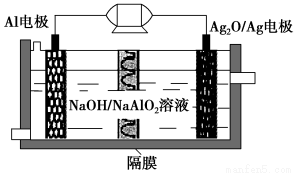
AΘ°ΙΛΉς ±’ΐΦΪΖΔ…ζΜΙ‘≠Ζ¥”Π
BΘ°Β±ΒγΦΪ…œ…ζ≥…1.08 g Ag ±Θ§Βγ¬Ζ÷–ΉΣ“ΤΒΡΒγΉ”ΈΣ0.01 mol
CΘ°AlΒγΦΪΒΡΖ¥”Π ΫΈΣAlΘ≠3eΘ≠ΘΪ4OHΘ≠=AlO2ΓΣΘΪ2H2O
DΘ°ΙΛΉς ±ΒγΫβ“Κ÷–ΒΡNaΘΪΆΗΙΐΗτΡΛ“ΤœρAlΒγΦΪ
D
ΓΨΫβΈωΓΩ’ΐΦΪΖΔ…ζΜΙ‘≠Ζ¥”ΠΘ§AΕ‘ΘΜ1.08 g“χΒΡΈο÷ ΒΡΝΩΈΣ0.01 molΘ§BΕ‘ΘΜ”…ΉήΖ¥”Π ΫΩ…÷ΣΘ§AlΒγΦΪ «ΗΚΦΪΘ§ΒγΦΪΖ¥”Π ΫΈΣAlΘ≠3eΘ≠ΘΪ4OHΘ≠=AlO2ΓΣΘΪ2H2OΘ§CΕ‘ΘΜ―τάκΉ””Π“Τœρ’ΐΦΪΘ§D¥μΓΘ

ΝΖœΑ≤αœΒΝ–¥πΑΗ
œύΙΊΧβΡΩ
ΕΧ÷ήΤΎ‘ΣΥΊRΓΔTΓΔQΓΔW‘Ύ‘ΣΥΊ÷ήΤΎ±μ÷–ΒΡœύΕ‘ΈΜ÷Ο»γΆΦΥυ ΨΘ§Τδ÷–TΥυ¥ΠΒΡ÷ήΤΎ–ρ ΐ”κΉε–ρ ΐœύΒ»ΓΘœ¬Ν–≈–Εœ≤Μ’ΐ»ΖΒΡ «(ΓΓΓΓ)
|
| R |
|
T | Q |
| W |
A.ΉνΦρΒΞΤχΧ§«βΜ·ΈοΒΡ»»Έ»Ε®–‘ΘΚR>Q
BΘ°ΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΥ°Μ·ΈοΒΡΥα–‘ΘΚQ<W
CΘ°‘≠Ή”ΑκΨΕΘΚT>Q>R
DΘ°Κ§TΒΡ―Έ»ή“Κ“ΜΕ®œ‘Υα–‘