��Ŀ����
����Ŀ����֪һЩ����֮�������ͼ��ʾ�����ǹ�ϵ��
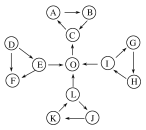
��ͼ��ÿ��С�����ε��������������ٺ���һ����ͬԪ�أ�
��DΪ���嵥�ʣ�OΪ���嵥�ʣ���������Ϊ���������
��C��ʹƷ����Һ��ɫ��Ҳ��ʹ�����ʯ��ˮ����ǣ�
��EΪ����ɫ�������L��Ӧ����O��
��L��J��ˮ��Һ������K��
��H�Ǻ���ɫ���壬���γ����ꡣ
��ش��������⣺
��1��E�ĵ���ʽΪ__���ṹ�к���__���������Ӽ����������Լ��������Ǽ��Լ�������д��E��F�����ӷ�Ӧ����ʽ___��
��2��K�Ļ�ѧʽΪ__��д��L��J��ˮ��Һ����K�Ļ�ѧ����ʽ___��
��3��I��Ũ��Һ����ǿ__�ԣ�����������������ԭ��������Ũ��Һ��C��Ӧ�Ļ�ѧ����ʽΪ__��
��4��A��C������ͬ��Ԫ����ɣ���A�Ļ�ѧʽΪ__��д��Cת��ΪA�Ļ�ѧ��Ӧ����ʽ__��
���𰸡�![]() ���Ӽ����Ǽ��Լ�
���Ӽ����Ǽ��Լ� ![]() NaHCO3
NaHCO3 ![]() ����
���� ![]() SO3
SO3 ![]()
��������
C��ʹƷ����Һ��ɫ��Ҳ��ʹ�����ʯ��ˮ����ǣ���C��SO2��A��SO3��B��H2SO4��
EΪ����ɫ�������E��Na2O2������DΪ���嵥�ʣ�����DΪNa��FΪNaOH��
Na2O2��L��Ӧ����O����O����̬���ʣ���OΪO2��LΪH2O��CO2��
����ΪL��J��ˮ��Һ������K����ȷ��L��CO2��J��NaCO3��KΪNaHCO3��
H�Ǻ���ɫ���壬���γ����꣬��H��NO2����IΪHNO3��GΪNO���ݴ˽��
��1��Na2O2�Ⱥ��зǼ��Թ��ۼ��ֺ������Ӽ��������ʽΪ��![]() ��E��F�ķ���ʽΪ
��E��F�ķ���ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() �����Ӽ����Ǽ��Լ���
�����Ӽ����Ǽ��Լ���![]() ��
��
��2��KΪNaHCO3��L��J����K�ķ���ʽΪ��![]() ���ʴ�Ϊ��NaHCO3��
���ʴ�Ϊ��NaHCO3��![]() ��
��
��3��IΪHNO3������ǿ�����ԣ��ܽ������������������ᣬͬʱŨ���ᱻ��ԭ��NO2���䷽��ʽΪ��![]() ���ʴ�Ϊ��������
���ʴ�Ϊ��������![]() ��
��
��4��A�����������������ڴ����ͼ��ȵ������·�Ӧ��������������ʽΪ��![]() ���ʴ�Ϊ��SO3��
���ʴ�Ϊ��SO3��![]() ��
��

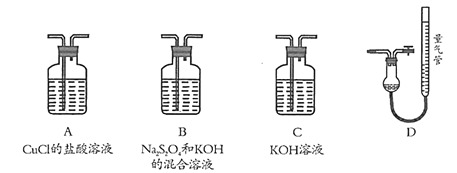
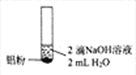
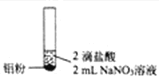
����Ŀ��ijͬѧ��������ʵ��![]() ��Һ��Ũ�Ⱦ�Ϊ
��Һ��Ũ�Ⱦ�Ϊ![]() ��
��
��� | �� | �� | �� |
ʵ�� |
|
|
|
���� | ������ɫ���� | ������ɫ����Һ���Ϸ���dz����ɫ | ������ɫ���� |
���ж�ʵ������Ľ��ͻ��Ʋⲻ��������
A.![]() �У�
�У�![]()
B.��������ʵ���жϣ�![]() ��������ǿ��ˮ
��������ǿ��ˮ
C.![]() �У�
�У�![]()
D.![]() ��ʹʪ��pH��ֽ������������
��ʹʪ��pH��ֽ������������![]()
����Ŀ����¯���������з�����Ӧ�� ![]() Fe2O3(s)��CO(g)
Fe2O3(s)��CO(g)![]()
![]() Fe(s)��CO2(g)���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����ұ�������˵����ȷ���ǣ� ��
Fe(s)��CO2(g)���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����ұ�������˵����ȷ���ǣ� ��
�¶�T/�� | 1000 | 1150 | 1300 |
ƽ�ⳣ��K | 4.0 | 3.7 | 3.5 |
A. �ɱ������ݿ��жϸ÷�Ӧ����Ӧ������������������������
B. 1000����Fe2O3��CO��Ӧ��t min�ﵽƽ��ʱc(CO) =2��10-3 mol/L������CO2��ʾ�÷�Ӧ��ƽ������Ϊ2��10��3/t mol��L��1��min��1
C. Ϊ��ʹ�÷�Ӧ��K��������������������ʱ������c(CO)
D. ������������ʱ������Fe2O3��������������Ч��������β����CO�ĺ���