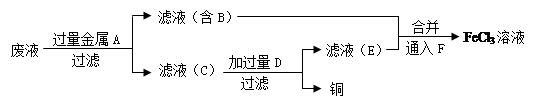
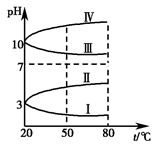
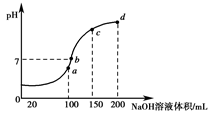
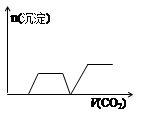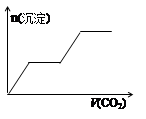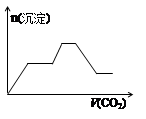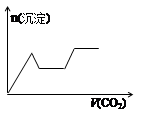��Ŀ����
�����������ʣ���Na2CO3 ��ͭ ���Ȼ��� ��CO2 ��NaHSO4 ��Ba(OH)2 �������������� �ఱˮ ��ϡ���� ��KI
��1�������ʵķ������д����Ŀհ״�(�����ʱ��)
| ����� | ����� | �� | �ǵ���� | ����� |
| ���ڸ��� ������ | | | | |
��2������ij������������Һ�пɷ������ӷ�Ӧ��H����OH��= H2O��д������һ�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽ ��
��3�����ʢ��ϡ��Һ�ڿ����б����������������Һ����ɫ����Ӧ�����ӷ���ʽΪ�� ��
��1���٢ۢݢޢ� �٢ݢ� �� �ߢ�� (��С�����©ѡ����ѡ�����÷�)
��2��Ba(OH)2+2HCl = BaCl2+2H2O�� Ba(OH)2+2HNO3 = Ba(NO3)2+2H2O
��3��O2 + 4I�� + 2 H2O = 2I2 + 4OH��
���������������2���������ӷ�ӦH����OH��= H2OӦΪǿ���ǿ�Ӧ���Ҳ������ɳ���������Ȳ������Ӧ���Ȼ��⡢ϡ������Ba(OH)2��Ӧ����3�������Ӿ��н�ǿ�Ļ�ԭ�ԣ����������䱻����Ϊ�ⵥ�ʣ���ҺΪ���Ի���������ʽΪO2 + 4I�� + 2 H2O = 2I2 + 4OH����
���㣺�������ʷ�������ӷ���ʽ��д���й����⡣

 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�����˵������ȷ����
| A�����⻯ѧ�����������������ꡱ���γɶ��뵪���������й� |
| B�����ݷ�ɢ����ֱ����С���Խ���ɢϵ��Ϊ��Һ���������Һ |
| C��SiO2������������ά���������ȶ���������ǿ�ᡢǿ�� |
| D����������ͷ���ijЩ����Ԫ�ص����ʵ�չ�� |
���������г��õ�����������������ᡱָ���ᡢ��������ᣬ�����ָ�ռ�ʹ��
��1�������ʵķ���Ƕȿ�����ǡ����һ��������________�����������ƣ���
��2�������ᡱ�롰���֮��ķ�Ӧ�����û�ѧ����ʽ��ʾ�������������ʱ�� ���������ӷ���ʽ��ʾȴֻ����������д�����������ӷ���ʽ���������ʱ��_____________________________��______________________��
��3�������ᡱ�������ܽ�����ͽ�����������п�״�����ڳ���ʱ��ȫ����������Ũ�������________��
| A��Au | B��Cu |
| C��Al | D��Fe |
��________����Na2CO3����________����NaHCO3��
��������ʮ�����ʣ���H2 ���� ��CuO ��CO2 ��H2SO4 ��Ba(OH)2 �ߺ��ɫ����������Һ�� �ఱˮ ��ϡ���� ��Al2(SO4)3
��1�������ʵķ������д����Ŀհ״���
| ����� | | ������ | | | ����� |
| ���ڸ�������� | �� | | ��� | �� | |
 H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ ��
H2O�������ӷ�Ӧ��Ӧ�Ļ�ѧ����ʽΪ ����3������ˮ�еĵ��뷽��ʽΪ ��17.1g������ˮ���250mL��Һ��SO42-��������Ϊ ��SO42-�����ʵ���Ũ��Ϊ ��
��4�������Ģ�ͨ�����Һ�з�Ӧ�����ӷ���ʽΪ ��
��5������ᷢ����Ӧ�Ļ�ѧ����ʽΪ��Al + 4HNO3 = Al(NO3)3 + NO�� + 2H2O���÷�Ӧ���������� ���ѧʽ������ԭ���������������ʵ���֮���� ������5.4g Al������Ӧʱ��ת�Ƶ��ӵ����ʵ���Ϊ ��
�÷�Ӧ�����ӷ���ʽΪ ��
�������ӷ���ʽ��ȷ���ǣ� ��
| A������ʯ��ˮ�еμ�ϡ���Ca(OH)2+2H+��Ca2++2H2O |
| B��ʵ�����ô���ʯ��ϡ������ȷCO2:2H++CO32-= CO2��+H2O |
| C������ͭ��Һ������������Һ��Ӧ��Ba2++SO42-=BaSO4���� |
| D��������SO2��NaOH��Һ��Ӧ��SO2+OH-=HSO3- |