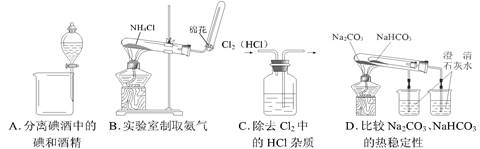
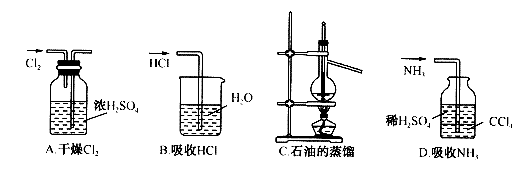
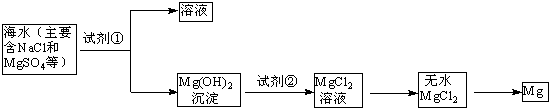
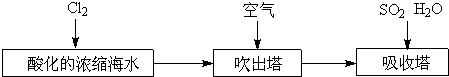
��Ŀ����
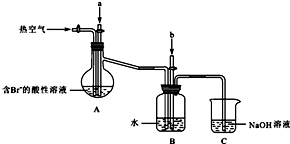
��2008�걱���������ۣ�27��(3)��ͼʾװ���Ʊ�NO����֤�仹ԭ�ԡ���������Ҫ������
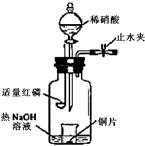
a������ƿ��ע��������NaOH��Һ����ʢ��ͭƬ��С�ձ�����ƿ�С�
b���ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c�������׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е�������ϡ���ᡣ
�ٲ���c��ȱ�ٵ�һ����Ҫ������___________________��
�ں��׳��ȼ�յIJ�����NaOH��Һ��Ӧ�����ӷ���ʽ�� ____________________________________________________________________��
�۲���c����ϡ������ձ��е�������_____________________________________
______________________________________________________________________����Ӧ�����ӷ���ʽ��____________________________________________________��

a������ƿ��ע��������NaOH��Һ����ʢ��ͭƬ��С�ձ�����ƿ�С�
b���ر�ֹˮ�У���ȼ���ף�����ƿ�У����ý�����
c�������׳��ȼ�գ�һ��ʱ����Һ©�����������ձ��е�������ϡ���ᡣ
�ٲ���c��ȱ�ٵ�һ����Ҫ������___________________��
�ں��׳��ȼ�յIJ�����NaOH��Һ��Ӧ�����ӷ���ʽ�� ____________________________________________________________________��
�۲���c����ϡ������ձ��е�������_____________________________________
______________________________________________________________________����Ӧ�����ӷ���ʽ��____________________________________________________��
��3���ٴ�ֹˮ�У�ͨ��������������P2O5+6OH-=2PO43��+3H2O����CuƬ���ܽ⣬����ɫ���ݲ�������Һ����ɫ��Ϊ��ɫ��3Cu+8H++2NO3��=3Cu2++2NO��+4H2O��
�Խ̲�ͭ��ϡ���ᷴӦʵ�����Ϊ���壬�ü���ƿ������ֹˮ�еĵ�������ؽ��������������������γ������ġ���ѧ��ʵ����ơ�ʵ��ԭ�������ú���ȼ������O2������ͭ��ϡ���ᷴӦ�Ʊ�NO���۲�����CuƬ���ܽ⣬����ɫ���ݲ�������Һ����ɫ��Ϊ��ɫ����Ӧ�����ӷ���ʽΪ��3Cu+8H++2NO3��=3Cu2++2NO��+4H2O��Ȼ���ֹˮ�У��ÿ��������װ�ã���������ɫ���֣�������2NO+O2=2NO2��ȷ��NO�л�ԭ�ԡ��ʲ���c��ȱ�ٵ�һ����Ҫ�����Ǵ�ֹˮ�У�ͨ������������

��ϰ��ϵ�д�
 ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ