��Ŀ����
ѡ���Դ���ԭ(SCR)�����д����������½�NOx ת��ΪN2��H2O������Ŀǰ�������᳧����β���������ձ���õ�һ�ַ�����

ijУ�ס���������ѧ��ȤС������֤NO�ܱ�������ԭ��������ת���ʡ�
��һ����������ȡ�����Ǹ���ͭ����
�Ǹ���ͭ��Adkin�������Ǽ��õ�NO����ԭ�Ĵ�������ͭ���ĸ���������ɷֲ��̶����磺CuO��Cr2O3�ȣ�ͳ��Ϊ�Ǹ���ͭ������ͬѧȡһ��������ͭ��Һ�������������ᱵ���ȶ������� �ظ������Һ�백ˮ���õ�����ɫ������ ��������[���ⶨΪ����ʽ����ͭ泥�CuNH4(OH)CrO4 )]���ˡ�ϴ�ӣ�80����12h�� ����ա�
��1�������õ��IJ��������У� �� ��ѡ����ţ���ͬ���������õ��������У� �� ��
G�������� H��©�� I��������������������ȥ��
��2��CuNH4(OH)CrO4��295��ֽ����ɸ��ϵ��������������������ˮ���÷�Ӧ�Ļ�ѧ����ʽ: �� ��
�������������ü����Ƶô������������̽���ʵ�顣
�Իش��������⣺
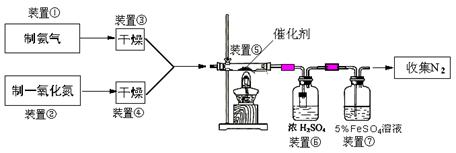
��3������ȡ������Aװ�ã�������Ӧ�Ļ�ѧ����ʽΪ�� �� ��
����Bװ����ȡ���������Һ©������ƿ��ʢ�ŵ�ҩƷ�ֱ��ǣ� �� ��

��4����ͼCװ����ȡNOʱ���ÿɳ鶯��ͭ˿���ŵ��ǣ� �� ��
��5��װ�âߵ����ÿ������� �� ��
��6��������װ�âݵ�NO��2688mL��������Ϊ��״������ͬ������������������ռ�����״����2016mLN2����NO��ת����Ϊ�� �� ��

ijУ�ס���������ѧ��ȤС������֤NO�ܱ�������ԭ��������ת���ʡ�
��һ����������ȡ�����Ǹ���ͭ����
�Ǹ���ͭ��Adkin�������Ǽ��õ�NO����ԭ�Ĵ�������ͭ���ĸ���������ɷֲ��̶����磺CuO��Cr2O3�ȣ�ͳ��Ϊ�Ǹ���ͭ������ͬѧȡһ��������ͭ��Һ�������������ᱵ���ȶ������� �ظ������Һ�백ˮ���õ�����ɫ������ ��������[���ⶨΪ����ʽ����ͭ泥�CuNH4(OH)CrO4 )]���ˡ�ϴ�ӣ�80����12h�� ����ա�
��1�������õ��IJ��������У� �� ��ѡ����ţ���ͬ���������õ��������У� �� ��
| A�������� | B��ʯ���� | C�������� | D���ձ� E������ǯ F���ƾ��� |
��2��CuNH4(OH)CrO4��295��ֽ����ɸ��ϵ��������������������ˮ���÷�Ӧ�Ļ�ѧ����ʽ: �� ��
�������������ü����Ƶô������������̽���ʵ�顣

�Իش��������⣺
��3������ȡ������Aװ�ã�������Ӧ�Ļ�ѧ����ʽΪ�� �� ��
����Bװ����ȡ���������Һ©������ƿ��ʢ�ŵ�ҩƷ�ֱ��ǣ� �� ��

��4����ͼCװ����ȡNOʱ���ÿɳ鶯��ͭ˿���ŵ��ǣ� �� ��
��5��װ�âߵ����ÿ������� �� ��
��6��������װ�âݵ�NO��2688mL��������Ϊ��״������ͬ������������������ռ�����״����2016mLN2����NO��ת����Ϊ�� �� ��
��1��D��G��H �� C��E��F��G��I����ѡ��ѡ�������֣���
��2��2Cu(OH)NH4CrO4 Cr2O3��2CuO+N2��+5H2O������д��������ƽ�����֣���
Cr2O3��2CuO+N2��+5H2O������д��������ƽ�����֣���
��3��2NH4Cl+Ca(OH)2 CaCl2+2NH3�� +2H2O��Ũ��ˮ����ʯ��(��ʯ�һ��������ƹ���)
CaCl2+2NH3�� +2H2O��Ũ��ˮ����ʯ��(��ʯ�һ��������ƹ���)
��4����Ӧ������ʱ��ͣ���������㡢����ʹ�á���ԼҩƷ��
��5������δ��Ӧ��NO
��6��90%
��2��2Cu(OH)NH4CrO4
 Cr2O3��2CuO+N2��+5H2O������д��������ƽ�����֣���
Cr2O3��2CuO+N2��+5H2O������д��������ƽ�����֣�����3��2NH4Cl+Ca(OH)2
 CaCl2+2NH3�� +2H2O��Ũ��ˮ����ʯ��(��ʯ�һ��������ƹ���)
CaCl2+2NH3�� +2H2O��Ũ��ˮ����ʯ��(��ʯ�һ��������ƹ���)��4����Ӧ������ʱ��ͣ���������㡢����ʹ�á���ԼҩƷ��
��5������δ��Ӧ��NO
��6��90%
���ڰѻ���ʵ��֪ʶ�������龰�dz������ⷽʽ���������ڼ��⣬��1����3�����ʿ�ֱ�ӻش𣻣�2������Ҫ�õ��龰��CuO��Cr2O3��Ϊ��Ӧ�IJ�����������ֿ�����������ԭ��Ӧ�����һ�ʽⷨ�����õ�Ԫ���غ㣺�ɷ�Ӧ����ʽ4NH3+6NO=5N2+6H2O��֪��������2016mL��0.09mol N2ʱ����ҪNO 0.108mol
��NOΪ0.12 mol������ת����Ϊ90%��
��NOΪ0.12 mol������ת����Ϊ90%��

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ

