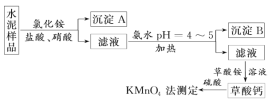
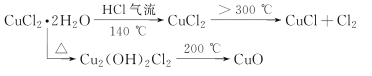��Ŀ����
����Ŀ��(1)A��һ����������ʽΪC4H8O2��A�����ɴ�B����C����������Ӧ�õ���B�����ɵ�C����
��д�����л�����Ľṹ��ʽ��
B______________________��C________________________________________��
��д��A�ڼ���������ˮ��Ļ�ѧ����ʽ��________________________________________��
��A�л���������B��C����ȥCѡ�õ��Լ���________�����뷽����_______________��
��B��C��������Ӧ��A��ˮ�ⷴӦ�ж��õ����ᣬ�����÷ֱ���________(����ĸ)��
a����������ˮ��������
b����������ˮ��������
c����������
d����ˮ��������
(2)�����������£�CH3CO18OC2H5��ˮ�������________��
(3)����ֲ�����е�������[CH3(CH2)4��CH=CH��CH2��CH===CH��(CH2)7COOH]�����ܵ͡����й����������˵������ȷ����________(����ĸ)��
a��һ��������������ͷ���������Ӧ
b������NaOH��Һ��Ӧ
c����ʹ����KMnO4��Һ��ɫ
d��1 mol�������������4 mol Br2�����ӳɷ�Ӧ
(4)�л���![]() ��һ������������������ˮ���л�ѧ���仯���ص�����жϣ�������������������ˮ������3�������ʣ��ṹ��ʽ�ֱ�Ϊ____________________��____________________________________________________��________��
��һ������������������ˮ���л�ѧ���仯���ص�����жϣ�������������������ˮ������3�������ʣ��ṹ��ʽ�ֱ�Ϊ____________________��____________________________________________________��________��
(5)�л���W(![]() )�����ںϳ�ά������ҩ����л���ķ���ʽΪ________������������������ŵ�����Ϊ______________��ˮ��Һ��l mol W����______ mol NaOH��ȫ��Ӧ�������к���______�ֲ�ͬ��ѧ��������ԭ�ӡ�
)�����ںϳ�ά������ҩ����л���ķ���ʽΪ________������������������ŵ�����Ϊ______________��ˮ��Һ��l mol W����______ mol NaOH��ȫ��Ӧ�������к���______�ֲ�ͬ��ѧ��������ԭ�ӡ�
���𰸡�CH3CH2OH CH3COOH CH3COOCH2CH3��NaOH��CH3COONa��CH3CH2OH ����Na2CO3��Һ ��Һ b CH3COOH��C2H518OH d  HOCH2CH2OH
HOCH2CH2OH ![]() C6H10O3 �ǻ������� 1 4
C6H10O3 �ǻ������� 1 4
��������
(1)A��һ����������ʽΪC4H8O2��A�в����Ͷ�=![]() =1������Ϊ����һԪ����A�����ɴ�B����C����������Ӧ�õ���B�����ɵ�C��˵��B��C��̼ԭ�Ӹ�����ȣ���AΪCH3COOCH2CH3��BΪCH3CH2OH ��CΪCH3COOH���ݴ˷������
=1������Ϊ����һԪ����A�����ɴ�B����C����������Ӧ�õ���B�����ɵ�C��˵��B��C��̼ԭ�Ӹ�����ȣ���AΪCH3COOCH2CH3��BΪCH3CH2OH ��CΪCH3COOH���ݴ˷������
(2)��ˮ����������ʹ���
(3)�����Ậ̼̼˫�����Ȼ������ϩ������������ʷ�����
(4)��������ˮ����ɣ�������������ˮ����������ʹ�������
(5)�����л���Ľṹ��ʽ�жϷ���ʽ�����л����к����ǻ������������жϣ�
��ͨ�����Ϸ���֪��A��B��C�Ľṹ��ʽ�ֱ�ΪCH3COOCH2CH3��CH3CH2OH��CH3COOH��
��A�ڼ���������ˮ�������Ҵ��������ƣ��÷�Ӧ�Ļ�ѧ����ʽΪCH3COOCH2CH3��NaOH��CH3COONa��CH3CH2OH��
����ȥCH3COOCH2CH3�е�CH3CH2OH��CH3COOH�����Լ��뱥��̼������Һ�������ܽ�CH3CH2OH������CH3COOH������CH3COOCH2CH3���ܽ�ȣ�CH3COOCH2CH3�뱥��̼������Һ�ֲ㣬�ʿ��Բ��÷�Һ�ķ������룻
��CH3CH2OH��CH3COOH��ȡCH3COOCH2CH3ʱ��Ũ��������������ˮ����CH3COOCH2CH3ˮ��ʱŨ�������������ʴ�Ϊb��
(2)��������������ˮ�����Ϊ����ʹ������������Ӧ��ԭ�����������ǻ������������Ĺ��ɿɵã�18O�����ڴ�����CH3COOH��C2H518OH��
(3)a��������ṹ�к����Ȼ��������DZ��������׳ƣ��������к����ǻ�������������ܹ��������һ�������·���������Ӧ����a��ȷ��
B��������ṹ�к����Ȼ����ܹ����������Ʒ�������кͷ�Ӧ����b��ȷ��
C��������ṹ�к���̼̼˫�����ܹ������Ը��������Һ�������������ʹ���Ը��������Һ��ɫ����c��ȷ��
D��1mol�������к���2mol̼̼˫��������1mol�����������2molBr2�����ӳɷ�Ӧ����d����
��ѡd��
(4)�����ʽṹ��ʽ�к���2���������ɷ���ˮ�⣬����������Ӧ���������ǻ������������Ĺ��ɿɵã�������������������ˮ�����ɵ����ʵĽṹ��ʽ�ֱ�ΪHOCH2CH2COOH��HOCH2CH2OH��CH3COOH��
(5)����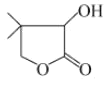 ��֪��ÿ���յ㣨�ڵ㣩Ϊ̼ԭ�ӣ�ÿ��̼ԭ���γ�4�����ۼ������㻯ѧ������ԭ�Ӳ��룬��W�ķ����к���6��̼ԭ�ӣ�10����ԭ�Ӻ�3����ԭ�ӣ��ʸ��л���ķ���ʽΪC6H10O3�������к��еĹ��������ǻ�������������W���Ӻ���1������������ˮ�������Ȼ��ܹ����������Ʒ�Ӧ����1molW����1molNaOH��ȫ��Ӧ�����ݵ�Ч��ԭ��ԭ��ͬһ̼ԭ���ϵ�H��ͬ���������ϵ�H��ͬ��������к���4�в�ͬ��ѧ��������ԭ�ӡ�
��֪��ÿ���յ㣨�ڵ㣩Ϊ̼ԭ�ӣ�ÿ��̼ԭ���γ�4�����ۼ������㻯ѧ������ԭ�Ӳ��룬��W�ķ����к���6��̼ԭ�ӣ�10����ԭ�Ӻ�3����ԭ�ӣ��ʸ��л���ķ���ʽΪC6H10O3�������к��еĹ��������ǻ�������������W���Ӻ���1������������ˮ�������Ȼ��ܹ����������Ʒ�Ӧ����1molW����1molNaOH��ȫ��Ӧ�����ݵ�Ч��ԭ��ԭ��ͬһ̼ԭ���ϵ�H��ͬ���������ϵ�H��ͬ��������к���4�в�ͬ��ѧ��������ԭ�ӡ�

����Ŀ����300 mL���ܱ������У��������۲�����һ������CO���壬һ�������·�����Ӧ��Ni(s)��4CO(g) ![]() Ni(CO)4(g)����֪�÷�Ӧƽ�ⳣ�����¶ȵĹ�ϵ�����ʾ��
Ni(CO)4(g)����֪�÷�Ӧƽ�ⳣ�����¶ȵĹ�ϵ�����ʾ��
�¶�/�� | 25 | 80 | 230 |
ƽ�ⳣ�� | 5��104 | 2 | 1.9��10��5 |
����˵����ȷ����(����)
A. ��������Ni(CO)4(g)�ķ�ӦΪ���ȷ�Ӧ
B. 25 ��ʱ��ӦNi(CO)4(g) ![]() Ni(s)��4CO(g)��ƽ�ⳣ��Ϊ0.5
Ni(s)��4CO(g)��ƽ�ⳣ��Ϊ0.5
C. ��80 ��ʱ�����ijʱ�̣�Ni(CO)4��COŨ�Ⱦ�0.5 mol/L�����ʱv��>v��
D. 80 ���ﵽƽ��ʱ�����n(CO)��0.3 mol����Ni(CO)4��ƽ��Ũ��Ϊ2 mol/L