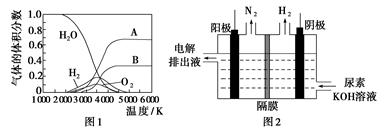
��Ŀ����
��100 mL NaOH��Һ��ͨ��CO2��ַ�Ӧ���ڼ�ѹ�ͽϵ��¶��£�С�ĵؽ���Һ���ɣ��õ���ɫ����M��ͨ�������̼�����V(��״����)��M������W�Ĺ�ϵ��ͼ
��ʾ����ش��������⣺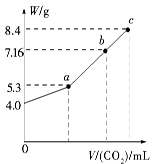
(1)b��ʱM����ɳɷ�Ϊ______________________��
(2)��Ҫʹb�����ɵ��ε�������Ϊ8.4 g����Ӧ��������Һ��ͨ�������̼________L(��״����)��
(3)�������ɵ�7.16 g�ε���Һ�м���һ������ij���ʣ���ַ�Ӧ��ѹ���������õ�������̼���ƹ���(�ᾧˮ)8.4 g��
����ֻ����0.03 molij���ʣ����������ʿ�����________��________��
����ֻ����0.06 molij���ʣ����������ʿ�����________��________��________��
(4)�����£�ͬŨ�ȵ�̼������Һ��̼��������Һ��pH
������7��������________��pH����������________________________��0.1 mol��L��1̼������Һ������Ũ�ȵĴ�С��ϵ��________����̼��������Һ����ε�������������Һ��������Ӧ�����ӷ���ʽΪ________��
(1)Na2CO3��NaHCO3��(2)0.448
(3)��Na2O��Na2O2����Na��NaOH��NaH
(4)Na2CO3������ͬ�����£�CO32����ˮ��������HCO3����ˮ������ǿ��c(Na��)��c(CO32��)��c(OH��)��c(HCO3��)��c(H��)��Ba2����2OH����2HCO3��=BaCO3����CO32����2H2O
����

���б�����ȷ����
| A��һ�ȼ���ĽṹʽCH3Cl |
| B���Ҵ��ķ���ʽCH3CH2OH |
C����������ģ�� |
D��Cl���ṹʾ��ͼ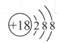 |
����������Ϊ36.5%��Ũ����(�ܶ�Ϊ1.16 g��cm��3)���Ƴ�1 mol��L��1��ϡ���ᡣ��ʵ���ҽ���Ҫ��������220 mL���Իش��������⣺
(1)����ϡ����ʱ��Ӧѡ������Ϊ mL������ƿ��
(2)��������Ҫ mLŨ���ᣬ����ȡʱ��ѡ��������Ͳ�е� ��
| A��5 mL | B��10 mL | C��25 mL | D��50 mL |
�ٵ�ϡ�͵�������¶�������һ�º��ز�����ע��250 mL����ƿ�С�
��������ƿ��С�ļ�����ˮ��Һ��������ƿ�̶���1��2 cmʱ�����ý�ͷ�ιܼ�����ˮ��ʹ��Һ��Һ����ƿ���Ŀ̶ȱ������С�
����ʢ������ձ���ע������ˮ�����ò�����������ʹ���Ͼ��ȡ�
��������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һȫ��ע������ƿ��
���������У���ȷ��˳����(�����) ��
(4)���������ƹ����У��øո�ϴ�ӽྻ����Ͳ����ȡŨ���ᣬ�����Ƶ�ϡ����Ũ���� (�ƫ�ߡ�����ƫ�͡�����Ӱ�족)����δ������ˮϴ���ձ��ڱںͲ�������δ��ϴ��Һע������ƿ�������Ƶ�ϡ����Ũ���� (�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��