��Ŀ����
5������������ֳ�Ī���Σ���dz��ɫ���壮���ڿ����б�һ���������ȶ����dz��õ�Fe2+�Լ���ijʵ��С�����ù�ҵ����м��ȡĪ���Σ����ⶨ����ɣ����ǽ���������ʵ�飮��Ī���ε���ȡ����ش��������⣮
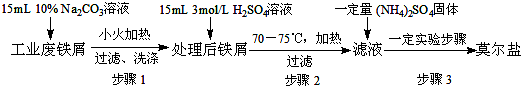
��1������м�к����������������Ʊ�ǰ��ȥ��������Fe2O3+6H+=2Fe3++3H2O��Fe+2Fe3+=3Fe2+�������ӷ���ʽ�ش𣩣�ʵ��ǰ���轫����м����̼������Һ����У��㵹��Һ�壬��ˮϴ����м��������������ѡ����װ����ɸò�������������Т٢ڢܢݢߣ����ţ���
������̨ �ڲ����� �۹��ƿ ��ʯ���� ���ձ� ��©�� �߾ƾ���
��2������2�м��ȷ�ʽˮԡ���ȣ��ֱ�Ӽ��ȡ��p��ˮԡ���ȡ���ɳԡ��������������м����ʣ��ʱ�������ȹ��ˣ���ԭ���Ƿ�ֹFe2+��������ͬʱ�ȹ��˿ɷ�ֹ���������Ծ�����ʽ����
II��Ī������ɵIJⶨ
�ٽ�Ħ���ε��º�ɺ�ȡ7.84g������100��ʧȥ�ᾧˮ��������Ϊ5.68g��
��ѡ��ͼ��ʾ�IJ���װ��������������������Ժ�����5.68g�������Aװ�õ���ƿ�У�������ƿ�м�������NaOHŨ��Һ��������ղ��������岢�����������Ϊ0.68g��
����A�м�������3%��H2 O2 ��Һ��������˳�������ϴ����������պ��������Ϊ1.6g��
��������ʵ��ش��������⣮
��3��������У�ѡ���װ����A��C��D������ţ���Aװ����δʹ�÷�Һ©����������NaOH��Һ�Է�Һ©��������������ĥ�ڸ�ʴ��ǿ��
��4����������ʵ�����ݼ��㣬Ħ������n��NH4+����n��Fe2+����n��SO${\;}_{4}^{2-}$����n��H2O��=2��1��2��6��
���� ��1������Һ���õ�������Ҫ����Ũ������ȴ�ᾧ�����ˡ�ϴ�Ӹ���ȹ��̣���������Һ�ļ��Ⱥ��ˣ���ϼ�������˻�������ѡ��������
��2��ˮԡ���ȱ��ڿ����¶ȣ����������ױ���������������ԭ�������ɵ�Fe3+��FeSO4���¶ȵ�ʱ�ܽ�Ƚ�С��
��3��Ī����������������Һ��Ӧ���ɰ���������ϡ�������գ�ע���ֹ�������������ƾ���ǿ��ʴ�ԣ�����������跴Ӧ��
��4����������ݿ�֪7.84gĦ������m��H2O��=7.84g-5.68g=2.16g�����ɵ�m��NH3��=0.68g��m��Fe2O3��=1.6g���Դ˿�ȷ��SO42-�������Լ�n��NH4+����n��Fe2+����n����SO42-����n��H2O���ı�ֵ��
��� �⣺��1�������к��������������Ʊ�ǰ��ȥ����������Һ��FeSO4��Һ�����������������������������������������������������ӷ���ʽΪ��Fe2O3+6H+=2Fe3++3H2O��Fe+2Fe3+=3Fe2+����������Һ�ļ��Ⱥ��ˣ���Ҫ�������У�����̨���ձ������������ƾ��ơ�ʯ�������ʲ���Ҫ���ƿ��©����
�ʴ�Ϊ��Fe2O3+6H+=2Fe3++3H2O��Fe+2Fe3+=3Fe2+���٢ڢܢݢߣ�
��2�������ʵ����Ҫ�¶�Ϊ��70��75�棬����ˮԡ���ȱ��ڿ����¶ȣ����������ױ��������������ɻ�ԭ�������ɵ�Fe3+�����ٲ����е�Fe3+���ʣ���������ȹ��˾ͻ���FeSO4•7H2O������
�ʴ�Ϊ��ˮԡ���ȣ���ֹFe2+��������ͬʱ�ȹ��˿ɷ�ֹ���������Ծ�����ʽ������
��3��Ī����������������Һ��Ӧ���ɰ��������ɰ������ü�ʯ�Ҹ���Գ�ȥˮ��Ȼ����ϡ������Dװ�������հ������Է�ֹ�������������ƾ���ǿ��ʴ�ԣ�����������跴Ӧ�������ע������������������Һ��
�ʴ�Ϊ��C��D�� NaOH��Һ�Է�Һ©��������������ĥ�ڸ�ʴ��ǿ��
��4����������ݿ�֪7.84gĦ������m��H2O��=7.84g-5.68g=2.16g��n��H2O��=$\frac{2.16g}{18g/mol}$=0.12mol��
���ɵ�m��NH3��=0.68g��m��Fe2O3��=1.6g��
��n��NH3��=$\frac{0.68g}{17g/mol}$=0.04mol��n��Fe2O3��=$\frac{1.6g}{160g/mol}$=0.01mol��
��m��NH4+��=0.04mol��18g/mol=0.72g��m��Fe2+��=0.02mol��56g/mol=1.12g��
��m��SO42-��=7.84g-2.16g-0.72g-1.12g=3.84g��
n��SO42-��=$\frac{3.84g}{96g/mol}$=0.04mol��
����n��NH4+����n��Fe2+����n����SO42-����n��H2O��=0.04mol��0.02mol��0.04mol��0.12mol=2��1��2��6��
�ʴ�Ϊ��2��1��2��6��
���� ���⿼�����ʵĺ����ⶨ��������ѧ���ķ�������������������ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�ע��������ʵ�����������ע�����

| A�� | H2O2�ĵ���ʽ��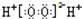 | |
| B�� | ��������ķ���ʽ��SiO2 | |
| C�� | ������ӵ����ģ�ͣ� | |
| D�� | ���ӽṹʾ��ͼ 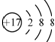 ���Ա�ʾ35Cl-��Ҳ���Ա�ʾ37Cl- ���Ա�ʾ35Cl-��Ҳ���Ա�ʾ37Cl- |
| A�� | ���������ͨ��ʢ������KMnO4��Һ��ϴ��ƿ | |
| B�� | ���������ͨ��ʢ��������ˮ��ϴ��ƿ | |
| C�� | �����������ͨ����������Ni�����ȵ������·�Ӧ | |
| D�� | ���������ͨ��ʢ��NaOH��Һ��ϴ��ƿ |
| A�� | �ձ����ƾ��ơ��Թܡ�����̨����Ȧ����©�� | |
| B�� | �ձ���©����������������̨����Ȧ������ֽ | |
| C�� | ��ֽ���ձ����ԹܼС�©���������� | |
| D�� | ��ֽ���Թܡ�©��������̨����Ȧ���������� |
| A�� | c��Na+����c��CO32-����c��HCO3-����c��OH-����c��H+�� | |
| B�� | c��Na+��-[c��CO32-��+c��HCO3-��]=c��OH-��-c��H+�� | |
| C�� | c��Na+����[c��CO32-��+c��HCO3-��+c��H2CO3��]=2��3 | |
| D�� | c��HCO3-��+2c��H+��+3c��H2CO3��=c��CO32-��+2c��OH-�� |
| A�� | K+��Cu2+��Na+��Cl- | B�� | H+��Na+��NO3-��CO32- | ||
| C�� | Ba2+��H+��NO3-��SO42- | D�� | Mg2+��Na+��OH-��SO42- |