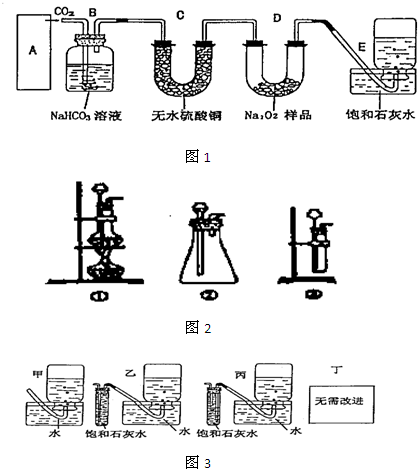
��Ŀ����
(һ)ijѧ��Ϊ�˲ⶨ���ֱ��ʵ�Na2SO3��Ʒ�Ĵ��ȣ����������ʵ�飺
��ش��������⣺
(1)д��Aװ���в������������ƣ��ƾ��ơ�____________��____________��
(2)ʵ�鿪ʼ��д��B�з�Ӧ�����ӷ���ʽ_____________________________________��
(3)C�е�������________________________��Eװ�õ�������______________________��
(4)����ͼ��ʾ��ȡһ������Na2SO3��Ʒ����Aװ�õ���ƿ�У�����������H2SO4��ȫ��Ӧ��Ȼ��B����ȫ��Ӧ�����Һ��������BaCl2��Һ��Ӧ�����ˡ�ϴ�ӡ�����ð�ɫ����
(5)�ڹ��˳���ʱ������Һ���ֻ��ǣ������Ҫ�ظ�___________����������ѧ��û���ظ��ò�����ⶨ�Ľ����___________(�ƫ�ߡ���ƫ�͡�����Ӱ�족)��
(6)Ҫʹ�ⶨ���ȷ����һ��װ�������Ա������ã��ڶ���Ӧ�ȵ�ȼ___________���ƾ���(��װ����ĸ)��
(��)�����йػ�ѧʵ��Ļ�����������ȫ֪ʶ������������ȷ����_________(�����)��
A.��������ƽ��ȡ
B.�������Թ��ڱڵı��ӣ����ü�Һϴ��
C.�ü�ʽ�ζ�����ȡ20.00 mL 0.100 0 mol��L-1�ĸ��������Һ
D.����������������л��е�NaNO3����
E.����Ũ�����Ũ����Ļ����ʱ����Ũ�����������������뵽Ũ�����У������Ͻ���
F.�и����ʱ�����������Ӽ�ȡ�����������ϵIJ���Ƭ�ϣ�С���õ��и�
G.ʵ��ʱ��������ȼ�ŵľƾ��ƣ���������ʪĨ���������
H.���ؽᾧ�����Գ�ȥ������л��е������Ȼ���
I.����ѧ������ͭ������ᾧˮ�����ⶨ����ʵ���У���������������Ҫ�Ĵ�
J.����һ��Ũ�ȵ���Һʱ��������ʱ��С�ļ�ˮ��������ƿ�Ŀ̶��ߣ�Ӧ�����õι���ȥ����IJ���
(һ)(1)Բ����ƿ����Һ©��
(2)Cl2+SO2+2H2O![]() 4H++2Cl-+
4H++2Cl-+![]()
(3)��ɫ�ʻ���ɫ ����δ��Ӧ���ж����壬��ֹ�ж�����Դ�������Ⱦ
(4)50.8% (5)���� ƫ�� (6)D
(��)ACFJ
������(һ)��������ԭ����A�в�����SO2��B�б�D�в�����Cl2����ΪH2SO4��ͨ���ⶨH2SO4�����������Na2SO3�Ĵ��ȡ�
(4)����ʱ�����ҩƷ�Ŵ���λ�ã�m(��Ʒ)+
m(��Ʒ)=![]() ��100%=50.8%��
��100%=50.8%��
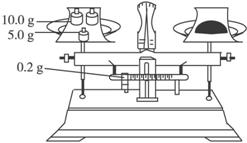
(��)������ƽֻ�ܾ�ȷ��С������һλ��A������ȡ���������ҺӦ����ʽ�ζ��ܣ�C��������Ӧ��ˮ���иF����J��Ӧ�������ơ�

| |||||||||||
(1)�±��г������������е�Ħ���������ڱ�����д����Ħ�����������������
| �������ͯ���� | �������������� | �л������� |
Ħ���� | �������� | ̼��� | �������� |
Ħ�������������(ָ�ᡢ��Ρ�������) |
|
|
|
(2)��������Ʋ⣬����Ħ�������ܽ�����___________��
(3)�����е�Ħ����̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ
![]()
![]()
��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��____________________________________________________________;
��____________________________________________________________;
��____________________________________________________________��
(4)��������ʯ��ʯΪԭ��(�����Լ���ѡ)�������һ���Ʊ�̼��Ƶ�ʵ�鷽��������(3)��ʾ�������ʵ�鷽��������ͼ��ʾ������
![]()
����Ƶķ����ŵ���____________________________________��
(5)�����������Ƿ���̼���ε�ʵ�鷽����____________________________________��
(6)ijѧ��Ϊ�˲ⶨһ����̼���ΪĦ������������̼��Ƶĺ��������ձ���ȡ�����������100.0 g�����ձ�����������������������ų�(��̼����⣬����������������ʲ��������ᷴӦ��������)�����ռ������������Ϊ22 g�������������������̼��Ƶ�����������(�����õ������ԭ��������C��12��O��16��Ca��40)