��Ŀ����
7��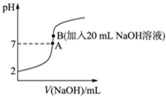 ��������Ҫ��һԪ�ᣬ���л�������Ӧ�ж���Ӧ�ã�����25��ʱ��pH=2�Ĵ��ᣮ��ش��������⣺
��������Ҫ��һԪ�ᣬ���л�������Ӧ�ж���Ӧ�ã�����25��ʱ��pH=2�Ĵ��ᣮ��ش��������⣺��1����������м������������ƹ��壬��ʱ��Һ��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$��С���������С�����䡱����
��2���ڴ����ϡ��Һ�У�ͨ���ı�����������ʹ����ĵ���Ȧ���CH3COOH���������ad����ʹ����ĵ���ƽ�ⳣ��Ka��CH3COOH���������a��
a�������¶�b������Һ�е���2��Ũ����
c����������CH3COONa����d����ˮϡ��
��3����20mL 0.2mol•L-1�Ĵ����еμ�0.2mol•L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ����ش��������⣺
��A��ʱc��Na+��=c��CH3COO-�����������������=������
��B��ʱ������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����c��Na+����c��CH3COO-����c��OH-����c��H+����
����������м���һ����NaOH��Һ�����û��ҺpH=6�������Һ��c��CH3COO-��-c��Na+��=��10-6-10-8��mol/L����дȷ���ݣ���
���ڴ��¶��£�����ĵ���ƽ�ⳣ��Ka��HF��Ϊ5.3��10-4��������λ��Ч���֣���
���� ��1�����ݴ����ƹ����ƽ��CH3COOH?CH3COO-+H+��Ӱ�������
��2��������ʵĵ������ȣ������ܹ��ٽ�������룬��ˮϡ�ʹ������̶�����
��3���پݵ���غ������
����B����ҺpH��7����c��H+����c��OH-�����ݴ˷�����
����Һ��c��CH3COO-��+c��OH-��=c��Na+��+c��H+����
����ͼ���֪0.2mol•L-1�Ĵ����pH=2����c��H+��=0.01mol/L���Դ˿ɼ������ƽ�ⳣ����
��� �⣺��1��������ƹ���������CH3COO-��c��CH3COO-������ʹ��ƽ��CH3COOH?CH3COO-+H+�����ƶ���C��H+����С��C��CH3COOH����������$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$��С��
�ʴ�Ϊ����С��
��2��a�������¶ȣ��������̶�������ƽ�ⳣ������
b������Һ�е���2��Ũ���ᣬ������Ũ�����������̶ȼ�С������ƽ�ⳣ�����䣻
c����������CH3COONa���壬���������Ũ��������ĵ���ƽ�������ƶ�������ƽ�ⳣ�����䣻
d����ˮϡ�ͣ��ٽ�������룬����ƽ�ⳣ�����䣬�ʴ�Ϊ��ad��a��
��3�����������Һ�еμ�NaOH�Ĺ����У�ʼ�մ���c��CH3COO-��+c��OH-��=c��Na+��+c��H+����A��ʱ��pH=7����c��OH-��=c��H+������c��CH3COO-��=c��Na+����
�ʴ�Ϊ��=��
���������Һ�еμ�NaOH�Ĺ����У�ʼ�մ���c��CH3COO-��+c��OH-��=c��Na+��+c��H+������B����ҺpH��7����c��H+����c��OH-������c��Na+����c��CH3COO-����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
���������Һ�еμ�NaOH�Ĺ����У�ʼ�մ���c��CH3COO-��+c��OH-��=c��Na+��+c��H+����pH=6����c��H+��=10-6mol/L����c��OH-��=10-8mol/L��c��CH3COO-��-c��Na+��=c��H+��-c��OH-��=��10-6-10-8��mol/L���ʴ�Ϊ����10-6-10-8����
����ͼ���֪0.2mol•L-1�Ĵ����pH=2����c��H+��=0.01mol/L����֪�����c��CH3COO-��=0.01mol/L��
K=$\frac{[C{H}_{3}CO{O}^{-}]•[{H}^{+}]}{[C{H}_{3}COOH]}$=$\frac{0.01��0.01}{0.2-0.01}$=5.3��10-4��
�ʴ�Ϊ��5.3��10-4��
���� ������Ҫ��������Ũ�ȴ�С�Ƚϣ�Ϊ��Ƶ���㣬������ѧ���ķ��������ͼ��������Ŀ��飬ע�����Ӱ��������ʵ�������أ�����غ�˼���������Ŀ���Ѷ��еȣ�

 ������������ϵ�д�
������������ϵ�д�| A�� | ����K=$\frac{{c}^{2}��Z��}{{c}^{2}��X��•c��Y��}$�����ŷ�Ӧ�Ľ��У�Z��Ũ�Ȳ�������X��Y��Ũ�Ȳ��ϼ�С��ƽ�ⳣ���������� | |
| B�� | ���¶Ȳ���ʱ������Ӧ���Ũ�ȣ�ʹK��С�������������Ũ�ȣ�ʹK���� | |
| C�� | ����ѹǿ��Kֵһ������ | |
| D�� | �¶ȷ����仯ʱ��������K ��ֵҲ�����仯 |
| A�� | n��Ca2+����С | B�� | c��Ca2+����С | C�� | n��OH-������ | D�� | c��OH-����С |
| A�� | 0.5 mol/L NaOH��Һ | B�� | 0.5 mol/L���� | ||
| C�� | 0.5 mol/L NH4Cl��Һ | D�� | 0.5 mol/L��ˮ |
| A�� | pH=12 | |
| B�� | pH=2 | |
| C�� | ��ˮ���������[H+]=1.0��10-2mol•L-1 | |
| D�� | ���ʵ����ʵ���Ũ��Ϊ0.01 mol•L-1 |
| A�� | Ũ�����ڹ����±�ƣ�˵��Ũ����ȶ��������ɵIJ��������Ũ���� | |
| B�� | ��ij��Һ�м����Ȼ�����Һ��ϡ���ᣬ�а�ɫ�������ɣ�˵������Һ��һ������SO42- | |
| C�� | �����£���ͭ����Ũ�����������Ա仯��˵��ͭ����Ũ�����жۻ� | |
| D�� | ����ʹʪ��ĵ⻯�ص�����ֽ������˵����������۷�Ӧ |