��Ŀ����
����Ŀ��Ϊ���о���ѧ��ӦA��B===C��D�������仯�����ijͬѧ�������ͼ��ʾװ�á�����ʢ��A���Թ��еμ��Լ�Bʱ������U�ι��м״�Һ���½��Ҵ�Һ���������Իش��������⣺
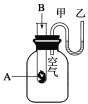
��1���÷�ӦΪ____________��Ӧ(��������������������)��
��2��A��B����������C��D��������____________(����������������)��
��3�������еĻ�ѧ��ͨ��______ת����______�ͷų�����
��4����Ӧ�ﻯѧ���������յ�����________(����������������)�������ﻯѧ���γɷų���������
��5���������ȼ����Ϊ890KJ/mol����д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ__________.
���𰸡���1������ ��2���� ��3����ѧ��Ӧ ���� ��4����
��5��CH4��g��+2O2��g��=CO2��g��+H2O��l�� ��H����890kJ��mol��1
��������
�����������1�����ڷ�����ӦA+B�TC+D��U���м״�Һ���½��Ҵ�Һ��������������������������������ʿ����жϸ÷�ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ���2������A+B�TC+D�ķ�ӦΪ���ȷ�Ӧ������A��B����������C��D���������ߣ��ʴ�Ϊ���ߣ���3����ѧ�仯���������ʺ������仯�������еĻ�ѧ��ͨ����ѧ��Ӧת���������ͷų������ʴ�Ϊ�����ܣ���4����ѧ��Ӧ�оɼ����������������¼����ɷų��������÷�ӦΪ���ȷ�Ӧ����Ӧ�ﻯѧ���������յ��������������ﻯѧ���γɷų�����������5���������ȼ����Ϊ890KJ/mol����д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪCH4��g��+2O2��g��=CO2��g��+H2O��l�� ��H����890kJ��mol��1

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�����Ŀ������Ϊ�����л�����й���Ϣ�����ݱ�����Ϣ�ش����⣮
A | B | C |
�������������һ�����ҵ�ʯ�ͻ���ˮƽ�� | ����ģ��Ϊ��
| ������Ҫ�ɷ� |
��1��A�ṹ��ʽ��_____________����������ʹ���Ը��������Һ��������Ȼ�̼��Һ��ɫ�����У����������ܷ����ķ�Ӧ��_______________���Ӧ���ͣ������巴Ӧ�Ļ�ѧ����ʽΪ_____________����������Ȼ�̼��Һ��������2.8gʱ�������������Ϊ__________��
��2����ҵ�ϣ�B��Դ��ú������Һ̬����ú���ͣ�B��Ũ���ᷢ��ȡ����Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ__________��
��3��C��������Ӧ�����ɶ��ȴ����д���÷�Ӧ�Ļ�ѧ����ʽΪ__________��
����Ŀ����ѧ�о��빤ҵ�����г����õ���Һ�еķ�Ӧ��
��1��25��ʱ��0.05 mol��L��1Ba��OH��2��Һ��pH�� ������Ba��OH��2��Һ��pH��2��HCl��Һ��ϣ������û����ҺpH��7����Ba��OH��2��Һ��HCl��Һ�������Ϊ ��
��2��CO2��ת�����л���ʵ��̼ѭ����CO2![]() CH3OH
CH3OH![]() HCOOH����
HCOOH����
25 ��ʱ�������������ƽ�ⳣ��������
��ѧʽ | HCOOH | H2CO3 | HClO |
����ƽ�ⳣ�� | 1.7��10��4 | K1��4.3��10��7 K2��5.6��10��11 | 3.0��10��8 |
�ش��������⣺
�������ӷ���ʽ��ʾHCOONa��Һ�ʼ��Ե�ԭ�� ��
�����ʵ���Ũ����Ϊ0.1 mol��L��1�������������ʣ�
a��Na2CO3 b��NaClO c��HCOONa d��NaHCO3 ��pH�ɴ�С��˳���� ����������
��3�������£���0.2 mol��L��1��HCOOH��0.1 mol��L��1��NaOH��Һ�������ϣ�������Һ��pH��7��˵�����û����Һ��HCOOH�ĵ���̶� HCOONa��ˮ��̶�������������������������С��������
��4������Cr2O72-�ķ�ˮ���Խϴ�ij������ˮ�к�5.00��10��3 mol��L��1��Cr2O72-��Ϊʹ��ˮ�ܴ���ŷţ������´�����Cr2O72-![]() Cr3����Fe3��
Cr3����Fe3��![]() Cr��OH��3��Fe��OH��3����������ķ�ˮ�в�����c��Fe3������2��10��13 mol��L��1���������Cr3����Ũ��Ϊ mol��L��1 ����֪��Ksp[Fe��OH��3]��4.0��10��38��Ksp[Cr��OH��3]��6.0��10��31����
Cr��OH��3��Fe��OH��3����������ķ�ˮ�в�����c��Fe3������2��10��13 mol��L��1���������Cr3����Ũ��Ϊ mol��L��1 ����֪��Ksp[Fe��OH��3]��4.0��10��38��Ksp[Cr��OH��3]��6.0��10��31����