��Ŀ����
��֪ij��Һ��ֻ����OH-��H+����c(Cl-)��c(![]() )��c(H+)��c(OH-)
)��c(H+)��c(OH-)
��c(![]() )��c(OH-)��c(Cl-)��c(H+)
)��c(OH-)��c(Cl-)��c(H+)
��c(![]() )��c(Cl-)��c(OH-)��c(H+)
)��c(Cl-)��c(OH-)��c(H+)
��c(Cl-) ��c(H+)��c(![]() )��c(OH-)
)��c(OH-)
��д���пհף�
(1)����Һ��ֻ�ܽ�һ�����ʣ����������_____________��������������Ũ�ȵĴ�С˳��Ϊ(�����)__________________________��
(2)���������ӵĹ�ϵ���Ϣۣ�������Ϊ_____________��
���������ӵĹ�ϵ���Ϣܣ�������Ϊ__________________________��
(3)��pH��ͬ��NH4Cl��Һ��HCl��Һϡ����ͬ�ı���������ͼ����ȷ����(��ͼ�����)__________________________��

(4)������Һ���������ȵ�ϡ����Ͱ�ˮ��϶��ɣ���ǡ�ó����ԣ�����ǰc(HCl)________c(NH3��H2O)(����ڡ���С�ڡ����ڡ�����ͬ)�����ǰ����c(H+)�ͼ���c(OH-)�Ĺ�ϵʽΪc(H+)_______c(OH-)��

(5)����ͼ��ʾ���ձ���ʢ�ŵ���ҺΪ���ᣬ��ͨ������G��ָ�뷢��ƫת����������______________��������Ӧʽ��______________________��
(1)NH4Cl ��
(2)NH4Cl��NH3��H2O NH4Cl��HCl
(3)B
(4)�� ����
(5) Zn 2H++ 2e-![]() H2��
H2��
������(1)��ֻ��һ�����ʣ�����OH-��H+����ˮ�ĵ���.����ΪNH4Cl������Һ������.
(2)���������ӵĹ�ϵ���Ϣ�c(![]() )��c(Cl-)��c(OH-)��c(H+)������Һ�Լ��ԣ��������Ե�Ψһ������NH3��H2O������Һ����Cl-�����ڼ���������ֻ������NH4Cl.
)��c(Cl-)��c(OH-)��c(H+)������Һ�Լ��ԣ��������Ե�Ψһ������NH3��H2O������Һ����Cl-�����ڼ���������ֻ������NH4Cl.
���������ӵĹ�ϵ���Ϣ�c(Cl-) ��c(H+)��c(![]() )��c(OH-)������Һ������.���������NH4Clˮ�����£�c(H+)ӦС��c(
)��c(OH-)������Һ������.���������NH4Clˮ�����£�c(H+)ӦС��c(![]() ).���������HCl���£���
).���������HCl���£���![]() ��������NH4Cl���������.
��������NH4Cl���������.
(3)ǿ���������ϡ��ʱ����Ҫ������pH�ı仯�����ϣ�ǿ����ʱ仯�ϴ����������Һ�д���ˮ��ƽ�⣬�仯�̶�С.
(4)�����кͲ�������NH4Clˮ��Һ�����ԣ�Ҫʹ��Һ�����ԣ���c(HCl)��c(NH3��H2O).����������ǿ�ᡢ���������c(H+)��c(OH-).
(5)���õ�һ���Ǹ�����ZnΪ����.

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�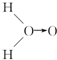 ��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѣ�
��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѣ�
 �ķе����
�ķе����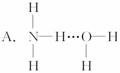

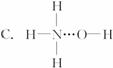

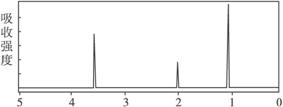
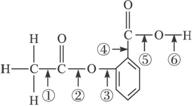
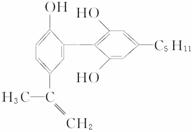


 �ӷ�Ӧʽ�� ��
�ӷ�Ӧʽ�� ��