��Ŀ����
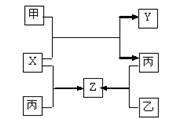
�������һ����Ҫ�ķǽ������ϣ��Ʊ��ߴ������Ҫ������ͼ��ʾ��

��֪SiHCl3����H2Oǿ�ҷ�Ӧ������һ�ֿ�ȼ�յĵ�������������ᣮ��ش��������⣺
��1��д���Ʊ��ֹ�Ļ�ѧ����ʽ______��
��2��������SiHCl3�ķ�Ӧ�У���ԭ���뻹ԭ�����������Ϊ______��
��3�������Ʊ����̱��������ˮ����������ˮ���ܷ����ķ�Ӧ��______��
��4�����й��ڹ輰�仯����������������______������ĸ���ţ�
A���������辧���й���ԭ�Ӽ��Թ��ۼ����
B�������ִ�ͨѶ�Ĺ��ά����Ҫ�ɷ��Ǹߴ��ȵĹ�
C������̼λ��ͬһ���壬�������ƣ�����Ȼ���й㷺����������̬�Ĺ�
D��SiO2��CO2�������������������ǿ���NaOH����Һ��Ӧ���������κ��ᷴӦ��
�⣺��1����������ͼ���������ԭ��Ӧ��֪��ӦΪ��SiO2+2C Si+2CO���ʴ�Ϊ��SiO2+2C
Si+2CO���ʴ�Ϊ��SiO2+2C Si+2CO��
Si+2CO��
��2����������ͼ��֪��ӦΪ��2Si+6HCl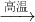 2SiHCl3+2H2����ԭ��ΪSi����ԭ����ΪH2��������֮��Ϊ��28��2������2��2��=14��1���ʴ�Ϊ��14��1��
2SiHCl3+2H2����ԭ��ΪSi����ԭ����ΪH2��������֮��Ϊ��28��2������2��2��=14��1���ʴ�Ϊ��14��1��
��3��������Ϣ��֪SiHCl3����H2Oǿ�ҷ�Ӧ������һ�ֿ�ȼ�յĵ�������������3SiHCl3+3H2O=H2SiO3+H2��+3HCl��ͬʱ̼����ˮ������Ӧ��C+H2O CO+H2��
CO+H2��
�ʴ�Ϊ��3SiHCl3+3H2O=H2SiO3+H2��+3HCl��C+H2O CO+H2��
CO+H2��
��4��A����������辧���й���ԭ�Ӽ��Թ��ۼ���ϣ���A��ȷ��
B������ά����Ҫ�ɷ��Ƕ������裬��B����
C�����Ԫ�صĴ�����ʽֻ�л���̬û������̬����C����
D����SiO2����HF�ᷴӦ����D����
��ѡ��BCD��
��������1����������ͼ���������ԭ��Ӧ��֪ʶ����д��
��2���ȸ�������ͼд������ʽ��Ȼ���ҳ���ԭ���뻹ԭ���ﲢ������ߵ������ȣ�
��3��������Ŀ��Ϣ�Լ�C����ˮ��Ӧ��
��4��A�����ݶ������辧���й���ԭ�Ӽ��Թ��ۼ���ϣ�
B�����ݹ��ά����Ҫ�ɷ֣�
C�����ݹ�Ԫ�صĴ�����ʽ��
D������SiO2����HF�ᷴӦ��
������������Ҫ�����˹輰�仯��������ʣ��ѶȲ����ݿα�֪ʶ������ɣ�
 Si+2CO���ʴ�Ϊ��SiO2+2C
Si+2CO���ʴ�Ϊ��SiO2+2C Si+2CO��
Si+2CO����2����������ͼ��֪��ӦΪ��2Si+6HCl
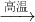 2SiHCl3+2H2����ԭ��ΪSi����ԭ����ΪH2��������֮��Ϊ��28��2������2��2��=14��1���ʴ�Ϊ��14��1��
2SiHCl3+2H2����ԭ��ΪSi����ԭ����ΪH2��������֮��Ϊ��28��2������2��2��=14��1���ʴ�Ϊ��14��1����3��������Ϣ��֪SiHCl3����H2Oǿ�ҷ�Ӧ������һ�ֿ�ȼ�յĵ�������������3SiHCl3+3H2O=H2SiO3+H2��+3HCl��ͬʱ̼����ˮ������Ӧ��C+H2O
 CO+H2��
CO+H2���ʴ�Ϊ��3SiHCl3+3H2O=H2SiO3+H2��+3HCl��C+H2O
 CO+H2��
CO+H2����4��A����������辧���й���ԭ�Ӽ��Թ��ۼ���ϣ���A��ȷ��
B������ά����Ҫ�ɷ��Ƕ������裬��B����
C�����Ԫ�صĴ�����ʽֻ�л���̬û������̬����C����
D����SiO2����HF�ᷴӦ����D����
��ѡ��BCD��
��������1����������ͼ���������ԭ��Ӧ��֪ʶ����д��
��2���ȸ�������ͼд������ʽ��Ȼ���ҳ���ԭ���뻹ԭ���ﲢ������ߵ������ȣ�
��3��������Ŀ��Ϣ�Լ�C����ˮ��Ӧ��
��4��A�����ݶ������辧���й���ԭ�Ӽ��Թ��ۼ���ϣ�
B�����ݹ��ά����Ҫ�ɷ֣�
C�����ݹ�Ԫ�صĴ�����ʽ��
D������SiO2����HF�ᷴӦ��
������������Ҫ�����˹輰�仯��������ʣ��ѶȲ����ݿα�֪ʶ������ɣ�

��ϰ��ϵ�д�
�����Ŀ
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣮
����д����̬��ԭ�ӵĺ�������Ų�ʽ______��
��NiO��FeO�ľ���ṹ���;����Ȼ�����ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO______�������������FeO��
��Ni��Fe��Co�Ƚ���������CO��Ӧ�γ�����Fe��CO��5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe��CO��5��������______��������ͣ�����λ����______��
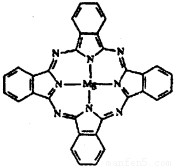
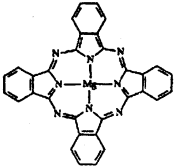
��2������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ����ͼ��������ͼ���ü�ͷ��ʾ����λ����
��3��CO��N2��Ϊ�ȵ����壮CO���ܼ��ܴ���N2���ܼ��ܣ���CO��N2���ײμӻ�ѧ��Ӧ�������±����ݣ�˵��CO��N2���õ�ԭ����______��
��4��CO��N2�����ж�����______���Ҽ���______���м���
��1��̫������ˮ���г�ʹ��һ�����������Ͻ������Ϊ���ռ���̫��������Ϳ�㣮
����д����̬��ԭ�ӵĺ�������Ų�ʽ______��
��NiO��FeO�ľ���ṹ���;����Ȼ�����ͬ��Ni2+��Fe2+�����Ӱ뾶�ֱ�Ϊ69pm��78pm�����۵�NiO______�������������FeO��
��Ni��Fe��Co�Ƚ���������CO��Ӧ�γ�����Fe��CO��5�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe��CO��5��������______��������ͣ�����λ����______��
��2������̪ݼ������ڹ�̫���ܵ��������Ҫ���ã�һ�ֽ���þ̪ݼ�����Ľṹ����ͼ��������ͼ���ü�ͷ��ʾ����λ����
��3��CO��N2��Ϊ�ȵ����壮CO���ܼ��ܴ���N2���ܼ��ܣ���CO��N2���ײμӻ�ѧ��Ӧ�������±����ݣ�˵��CO��N2���õ�ԭ����______��
| A-B | A=B | A��B | ||
| CO | ���� | 357.7 | 798.9 | 1071.9 |
| ���ܲ�ֵ | 441.2 273 | |||
| N2 | ���� | 154.8 | 418.3 | 941.7 |
| ���ܲ�ֵ | 263.6 523.3 | |||

�ס��ҡ��������ֳ������ʣ�X��Y��Z�dz������������֮��������ת����ϵ��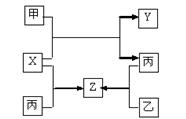
��֪���Ƕ����ڽ������ʣ��ҡ����Ƕ����ڷǽ������ʣ�X��Y��Z��ֻ��һ�������Ӿ��塣����˵������ȷ����
| A��X�Ǿ��м��Լ��ķǼ��Է��� | B��Z��ˮú������Ҫ�ɷ�֮һ |
| C����X�ķ�Ӧ�����ȷ�Ӧ | D���������������Ҫԭ�� |
 ��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����
��Դ���������Ϊ��Լ������ᾭ�÷�չ��ƿ����Խ��Խ��Ĺ��ҿ�ʼʵ�С�����ƻ���������̫������Դ��Ѱ�÷�չ���¶�����