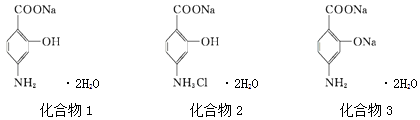
��Ŀ����
����Ŀ���ȡ�������Ļ���������������������������е���ϵ����ش��������⣺
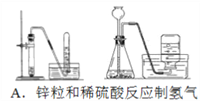
��1����仯ѧ�����ս����̿���Ҫ�ɷ���MnO2����Ũ�����ϼ��ȣ��������������Ƶ�����������Ӧ�Ļ�ѧ����ʽΪ��_________________________�����У���������_______����ʵ�����Ƶñ�״����4.48L��������ת�Ƶ��ӵ���ĿΪ_____����
��2���ڵ��Ļ������У�����ɫ���д̼�����ζ���ж�������___________����д��ѧʽ������д����������ˮ��Ӧ�Ļ�ѧ����ʽ________________________��
��3������ƽŨ�����ڼ���ʱ��̼������Ӧ�Ļ�ѧ����ʽ��____C+____H2SO4(Ũ)=__CO2��+_____SO2��+____H2O��
�ڸ÷�Ӧ�У�ŨH2SO4���ֳ�___________(���ˮ��������ˮ����������)�ԡ�
���𰸡� MnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O MnO2��������� 0.4NA ��2.408��1023 NO2 3NO2 + H2O=2HNO3 + NO _1__C+___2_H2SO4(Ũ)
MnCl2+Cl2��+2H2O MnO2��������� 0.4NA ��2.408��1023 NO2 3NO2 + H2O=2HNO3 + NO _1__C+___2_H2SO4(Ũ)![]() __1__ CO2��+__2___SO2��+__2__H2O ����
__1__ CO2��+__2___SO2��+__2__H2O ����
����������1��MnO2��Ũ�����Ϲ�������MnCl2��Cl2��H2O���Ա�������ԭ��Ӧ�и���ͼ�������
��2���ڵ��Ļ������У�����ɫ���д̼�����ζ���ж�������NO2��NO2��H2O��Ӧ����HNO3��NO��
��3���û��ϼ���������ƽ�����ݷ���ʽ����Ũ��������ʡ�
��1��MnO2��Ũ�����Ϲ�������MnCl2��Cl2��H2O����Ӧ�Ļ�ѧ����ʽΪMnO2+4HCl![]() MnCl2+Cl2��+2H2O���ڸ÷�Ӧ��MnԪ�صĻ��ϼ���+4�۽�Ϊ+2�ۣ���������MnO2��ClԪ�صĻ��ϼ���-1������0����ÿ����1molCl2ת��2mol������n��Cl2��=
MnCl2+Cl2��+2H2O���ڸ÷�Ӧ��MnԪ�صĻ��ϼ���+4�۽�Ϊ+2�ۣ���������MnO2��ClԪ�صĻ��ϼ���-1������0����ÿ����1molCl2ת��2mol������n��Cl2��=![]() =0.2mol��ʵ�����Ƶñ�״����4.48LCl2ת�Ƶ������ʵ���Ϊ0.2mol
=0.2mol��ʵ�����Ƶñ�״����4.48LCl2ת�Ƶ������ʵ���Ϊ0.2mol![]() 2=0.4mol��ת�Ƶ�����Ϊ0.4NA��0.4
2=0.4mol��ת�Ƶ�����Ϊ0.4NA��0.4![]() 6.02
6.02![]() 1023=2.408
1023=2.408![]() 1023��
1023��
��2���ڵ��Ļ������У�����ɫ���д̼�����ζ���ж�������NO2��NO2��H2O��Ӧ����HNO3��NO����Ӧ�Ļ�ѧ����ʽΪ3NO2+H2O=2HNO3+NO��
��3����Ӧ��CԪ�صĻ��ϼ���0������+4����1molCʧȥ4mol��������1molCO2��SԪ�صĻ��ϼ���+6�۽���+4����1molH2SO4��Ũ���õ�2mol��������1molSO2�����ݵ�ʧ�����غ㡢ԭ���غ㣬��ƽ�ķ���ʽΪ1C+2H2SO4��Ũ��![]() 1CO2��+2SO2��+2H2O���ڸ÷�Ӧ������+6�۵�Sȫ����ԭΪ+4�ۣ�ŨH2SO4������������
1CO2��+2SO2��+2H2O���ڸ÷�Ӧ������+6�۵�Sȫ����ԭΪ+4�ۣ�ŨH2SO4������������

����Ŀ�����Ȼ�����(S2Cl2)��һ����Ҫ�Ļ���ԭ��,����������,�ı��������ȷ�ճ�������Ӳ�����ʡ��������Ͽ�֪S2Cl2������������:
�������� | ���� | ɫ̬ | �ӷ��� | �۵� | �е� |
�綾 | ���ɫҺ�� | �ӷ� | -76�� | 138�� | |
��ѧ���� | ��300 ��������ȫ�ֽ� ��S2Cl2��Cl2 �������Ȼ�������Ӵ�,������ȼ�յ�Σ�� �����Ȼ���ˮ�ֽ����,�ų���ʴ������ | ||||
(1)��ȡ����S2Cl2
ʵ���ҿ�������������������110~140�淴Ӧ�Ƶ�S2Cl2��Ʒ�����������������SCl2��

������m ������Ϊ__________,װ��F ���Լ���������_________��
��װ������˳��: A![]() ______
______![]()
![]()
![]() E
E![]() D��
D��
��ʵ��ǰ��K1,ͨ��һ��ʱ��ĵ����ž�װ���ڿ�����ʵ�����ֹͣ���Ⱥ���ͨ��һ��ʱ��ĵ���,��Ŀ����_____________��
��Ϊ�����S2Cl2�Ĵ���,ʵ��Ĺؼ��ǿ��ƺ��¶Ⱥ�____________��
(2)����S2Cl2й©ʱӦ��ˮ��������ӷ�(����ɢ),��������������Һ,����Ҫ��й©���й©��ֱ����ˮ,��ԭ����______________��
(3)S2Cl2��ˮ������SO2��HCl��������,ijͬѧ���������ʵ�鷽�����ⶨ�û��������SO2�����������

��W��Һ������_____(����)��
a.H2O2��Һ b.KMnO4��Һ(�����ữ) c.��ˮ
�ڸû�������ж���������������Ϊ_________(�ú�V��m ��ʽ�ӱ�ʾ)��
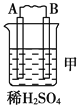
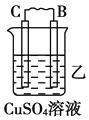
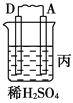
����Ŀ����.��A��B��C��D���ֽ������±���װ�ý���ʵ�顣
װ�� |
|
|
|
���� | ���۽���A�����ܽ� | C���������� | A����������� |
����ʵ������ش��������⣺
��1��װ�ü��и����ĵ缫��Ӧʽ��_______________��װ�����������ĵ缫��Ӧʽ��___________��
��2�����ֽ�����������ǿ������˳����_____________________________________��
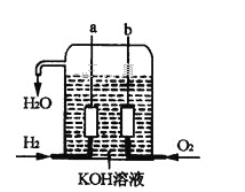
��.��������������ɴ���ʹ����һ�����ͷ���װ��������ȼ�ϵ�أ��乹����ͼ��ʾ������a��b�����缫���ɶ��̼Ȳ��ɣ��õ�صĸ�����ӦʽΪ_________________�����õ�ع���ʱ��Һ������1molH2O���������ϵ�·��ͨ�����ӵ����ʵ���Ϊ________mol��