��Ŀ����
���淴ӦN2(g)+3H2(g)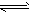 2NH3(g) ��H=-QKJ/mol(Q>0)���мס��������ݻ���ͬ�Ҳ�����ܱ���������������м���1molN2��3molH2����һ�������´ﵽƽ��ʱ�ų�����ΪQ1KJ������ͬ�����£����������м���2molNH3�ﵽƽ�����������ΪQ2KJ����֪Q1=4Q2��������������ȷ����
2NH3(g) ��H=-QKJ/mol(Q>0)���мס��������ݻ���ͬ�Ҳ�����ܱ���������������м���1molN2��3molH2����һ�������´ﵽƽ��ʱ�ų�����ΪQ1KJ������ͬ�����£����������м���2molNH3�ﵽƽ�����������ΪQ2KJ����֪Q1=4Q2��������������ȷ����
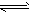 2NH3(g) ��H=-QKJ/mol(Q>0)���мס��������ݻ���ͬ�Ҳ�����ܱ���������������м���1molN2��3molH2����һ�������´ﵽƽ��ʱ�ų�����ΪQ1KJ������ͬ�����£����������м���2molNH3�ﵽƽ�����������ΪQ2KJ����֪Q1=4Q2��������������ȷ����
2NH3(g) ��H=-QKJ/mol(Q>0)���мס��������ݻ���ͬ�Ҳ�����ܱ���������������м���1molN2��3molH2����һ�������´ﵽƽ��ʱ�ų�����ΪQ1KJ������ͬ�����£����������м���2molNH3�ﵽƽ�����������ΪQ2KJ����֪Q1=4Q2��������������ȷ���� [ ]
A����A��ƽ��ʱ���������з�Ӧ���ת���������������
B���ﵽƽ��ʱ������NH3����������������
C���ﵽƽ����������м���0��2moLN2��0��6molH2��1��6molNH3��ƽ��������NH3�ķ����ƶ�
D�����е��Ȼ�ѧ��Ӧ����ʽΪ2NH3(g)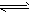 N2(g)+3H2(g)����H=+QKJ/mol(Q>Q2>0)
N2(g)+3H2(g)����H=+QKJ/mol(Q>Q2>0)
B���ﵽƽ��ʱ������NH3����������������
C���ﵽƽ����������м���0��2moLN2��0��6molH2��1��6molNH3��ƽ��������NH3�ķ����ƶ�
D�����е��Ȼ�ѧ��Ӧ����ʽΪ2NH3(g)
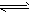 N2(g)+3H2(g)����H=+QKJ/mol(Q>Q2>0)
N2(g)+3H2(g)����H=+QKJ/mol(Q>Q2>0) A

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
���ڿ��淴ӦN2(g)+3H2(g) 2NH3(g)����H��0�������о�Ŀ�ĺ�ʾ��ͼ�������
2NH3(g)����H��0�������о�Ŀ�ĺ�ʾ��ͼ�������
|
A |
B |
C |
D |
|
�о�Ŀ�� |
ѹǿ�Է�Ӧ�� Ӱ�죨P2>P1�� |
�¶ȶԷ�Ӧ��Ӱ�� |
ƽ����ϵ����N2�Է�Ӧ��Ӱ�� |
�����Է�Ӧ�� Ӱ�� |
|
ͼʾ |
|
|
|
|
 2NO(g)�Ѵﵽƽ��״̬����(����)
2NO(g)�Ѵﵽƽ��״̬����(����) B(g)��C(g)��������������������£��ٳ���һ������A���壬A��ת���ʽ�����
B(g)��C(g)��������������������£��ٳ���һ������A���壬A��ת���ʽ����� 2NH3(g)��������Ũ�ȿ����ӻ���Ӱٷ������Ӷ�ʹ��Ӧ�������ӡ�
2NH3(g)��������Ũ�ȿ����ӻ���Ӱٷ������Ӷ�ʹ��Ӧ�������ӡ� 2NH3(g)��H<0�����¸�Ӱ�����ظı��ʾ��ͼ����ϵ��ǣ� ��
2NH3(g)��H<0�����¸�Ӱ�����ظı��ʾ��ͼ����ϵ��ǣ� ��



