��Ŀ����
�����£����и������ӻ������ָ����Һ���ܴ����������
| A�����ȳʺ�ɫ����Һ�У�MnO4����Al3����C2H5OH��SO42- |
| B��ˮ�������c(H��)��1��10��14 mol��L��1����Һ�У�Ca2����NH4����Cl����SiO32�� |
| C��c(H��)/c(OH��)��1012����Һ�У�NH4+��Al3����NO3-��ClO�� |
| D��c(NaHCO3)��0.1 mol��L��1����Һ�У�K����C6H5O����SO42-��CO32�� |
D
�������������A��ʹ���ȳʺ�ɫ����ҺΪ������Һ����ʱMnO4�����C2H5OH����������ʴ������ԭ��Ӧ�����ܴ������档����B���������£�ˮ���������c(H��)= 1��10��7 mol/L.����ˮ�������c(H��)��1��10��14 mol/L<1��10��7 mol/L,˵����Һ�����Ի���ԡ�����Һ�����ԣ�������Ӧ��H++ SiO32��=H2SiO3��������Һ�ʼ��ԣ�������Ӧ��NH4��+OH-=NH3+H2O�����Զ����ܴ������档����C. c(H��)/c(OH��)��1012, c(H��)��c(OH��)��10-14.��ʽ������⣬�ɵ�c(H��)=0.1mol����ҺΪ���ԣ���ʱ������Ӧ��H++ ClO��=HClO.���ܴ������档D. NaHCO3��ѡ���е����Ӳ��ᷢ���κη�Ӧ�����Դ������档��ȷ��
���㣺�������ӻ�����ܷ���������֪ʶ��

 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д���ѧ�г���������ͼ����ʾij�ֱ仯���̣����˷ֱ������е��ĸ�����ͼ��
�й���������ͼ��˵����ȷ���ǣ�
| A������ͼ�ٿ��Ա�ʾ��ѹ������ij��ѧƽ����ϵ���������������Ӧ������ʱ��ı仯 |
| B������ͼ�ڿ��Ա�ʾ��һ����������������Һ�еμ�һ��Ũ�ȵ�����ʱ��pH�ı仯 |
| C������ͼ�ۿ��Ա�ʾ��һ������������Һ�еμ�һ��Ũ�ȵ�����������Һʱ�IJ������������ʵ����仯 |
| D������ͼ�ܿ��Ա�ʾ�������ˮϡ��������Һ��c(H+)�ı仯 |
�������ӷ���ʽ��д��ȷ���ǡ�������
| A��Na2S2O3��Һ�м���ϡ���2S2O32�� + 4H+ = SO42�� + 3S�� + 2H2O |
B����ͭ���缫���CuSO4��Һ��2Cu2+ + 2H2O 2Cu + O2��+ 4H+ 2Cu + O2��+ 4H+ |
| C����H2O2�μӵ�����KMnO4��Һ�У�2MnO4��+H2O2+6H+=2Mn2+ +3O2 ��+4H2O |
| D������FeBr2��Һ��ͨ��������Cl2��2Fe2����Cl2=2Fe3����2Cl�� |
���л�����������������ʵ���
| A��BaSO4 | B��HCl | C��CO2 | D��H2O |
�������ӷ���ʽ�в���ȷ���ǣ�������
| A��������þ��ɫ���������Ȼ����Һ��Mg(OH)2��2NH4+��Mg2+��2NH3��H2O |
B��ͭƬ�ӵ�Դ������̼,���ӵ�Դ���������������ҺCu��2H��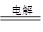 Cu2����H2�� Cu2����H2�� |
C������һ������Һˮ�⣺HPO42����H2O PO43����H3O�� PO43����H3O�� |
| D��Ư����Һ�м��Ȼ�����Һ�����������ɫ������ Fe3+��3ClO����3H2O��Fe(OH)3����3HClO |
����ɫ������Һ�п��Դ����������������
| A��H+��K+��Fe2+��NO3�� | B��OH����Cl����Na+��NH4+ |
| C��Mg2+��K+��Cl����NO3�� | D��Cu2+��NO3����OH����Cl�� |
���и���������ָ����ɢϵ��һ���ܴ����������
A��pH��0����Һ�У�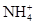 ��Fe3���� ��Fe3����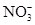 ��Cl�� ��Cl�� |
B��������Һ�У�Na����Mg2����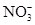 �� ��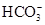 |
| C����������������H����K����Cl����S2�� |
D��ʹ��̪������Һ�У�Na����Ba2����Cl����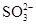 |
��֪���Ը��������Һ���Խ�FeSO4����������ʽΪ2KMnO4+10FeSO4+ 8H2SO4=K2SO4+2MnSO4+5Fe2(SO4)3+8H2O���ֽ�һ�����������ữ�ĸ��������Һ������������Һ��ϣ���ַ�Ӧ������������Һ�м���KI��Һ�������Һ�������ӵ����ʵ���������KI�����ʵ����ı仯��ϵ����ͼ��ʾ��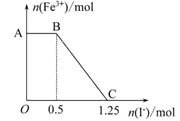
�������й�˵������ȷ����( )
| A��ͼ��AB����Ҫ�Ǹ�����غ͵⻯����Һ��Ӧ |
| B��ͼ��BC�η����ķ�ӦΪ2Fe3++2I-=2Fe2++I2 |
| C������OC�ε����ݿ�֪��ʼ����ĸ�����ص����ʵ���Ϊ0.25 mol |
| D����C���Ժ����Һ�м�������KSCN��Һ����Һ���ɫ |