��Ŀ����
����Ŀ�����������������ڸ����ı����γ�һ�������������ļ������̡�����һ�ְ취�ǽ�������Ʒ�����������ƺ�Ũ�������ƵĻ����Һ�м��ȵ�130�淴Ӧ�����������գ�
(1)�似�������������µĻ�ѧ����ʽ��
��____Fe+____NaNO2+____NaOH��____Na2FeO2+____H2O+____NH3����
��6Na2FeO2 + NaNO2 + 5H2O =3Na2Fe2O4 + NH3�� + 7NaOH��
��Na2FeO2 + Na2Fe2O4 + 2 H2O = Fe3O4 + 4 NaOH��
����ƽ����ʽ�٣����������ת�Ƶķ������Ŀ_____________��
(2)����������������Ӧʱ���ס��ҡ�����λͬѧ������������ǵĿ���������˵����ȷ����_____________��
��ͬѧ�������������в��������Ⱦ����
��ͬѧ��ֻҪ����NaNO2 ����ķ�Ӧ�������dz䵱��������ɫ��
��ͬѧ��ÿһ���������漰���Ļ�ѧ��Ӧ���ɹ�����������ԭ��Ӧԭ��
(3)���������̹����67.2������������£������ת��________mol��
(4)�����1mol Fe3O4����Ӧ������NaOH��������________������������������������________mol��
���𰸡�3 1 5 3 1 1 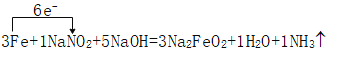 ��ͬѧ 18 ����
��ͬѧ 18 ���� ![]()
��������
(1)FeԪ�صĻ��ϼ���0����Ϊ+2�ۣ�NԪ�صĻ��ϼ���3�۽���Ϊ-3�ۣ���ϵ��ӡ�ԭ���غ������
(2)������Ⱦ�������٢���NԪ�صĻ��ϼ۽��ͣ�����û��Ԫ�صĻ��ϼ۱仯��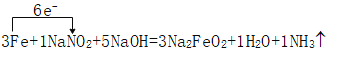
(3)���ð��������ʵ�����NԪ�صĻ��ϼ۱仯����ת�Ƶ��ӣ�
(4)��6Fe+2NaNO2+10NaOH=6Na2FeO2+2H2O+2NH3����6Na2FeO2+NaNO2+5H2O=3Na2Fe2O4+NH3��+7NaOH��֪������3mol Na2Fe2O4����3mol NaOH����3Fe+NaNO2+5NaOH=3Na2FeO2+H2O+NH3����֪������3mol Na2FeO2������5mol NaOH��������8mol NaOH����3Na2FeO2+3Na2Fe2O4+6H2O=3Fe3O4+12NaOH�У�����3mol Fe3O4������12mol NaOH��������3mol Fe3O4��������4mol NaOH��
(1)FeԪ�صĻ��ϼ���0����Ϊ+2�ۣ�NԪ�صĻ��ϼ���3�۽���Ϊ-3�ۣ��ɵ��ӡ�ԭ���غ��֪��ӦΪ3Fe+NaNO2+5NaOH=3Na2FeO2+H2O+NH3���������ű�ʾΪ:��
(2)������Ⱦ��������˵�����٢���NԪ�صĻ��ϼ۽��ͣ�����NaNO2����ķ�Ӧ�������dz䵱��������ɫ����˵����ȷ������û��Ԫ�صĻ��ϼ۱仯��������������ԭ��Ӧ��
(3)�а������ɵķ�Ӧ��������NaNO2~ NH3������һ��NH3��ת��6�����ӣ����������̹����67.2������������£������ת��Ϊ![]() =18mol��
=18mol��
(4)��6Fe+2NaNO2+10NaOH=6Na2FeO2+2H2O+2NH3����6Na2FeO2+NaNO2+5H2O=3Na2Fe2O4+NH3��+7NaOH��֪������3mol Na2Fe2O4����3mol NaOH����3Fe+NaNO2+5NaOH=3Na2FeO2+H2O+NH3����֪������3mol Na2FeO2������5mol NaOH��������8mol NaOH����3Na2FeO2+3Na2Fe2O4+6H2O=3Fe3O4+12NaOH�У�����3mol Fe3O4������12mol NaOH��������3mol Fe3O4��������4mol NaOH�����������1molFe3O4��Ӧ������NaOH�����������![]() mol NaOH��
mol NaOH��

����Ŀ����ͼ�����÷�ͭм�������������Ʊ�����������ͭ���壩�����̡�
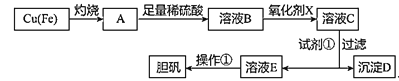
��Һ�б��������� | Fe3+ | Fe2+ | Cu2+ |
��ȫ���������������ʱ����Һ��pH | ��3.7 | ��6.4 | ��4.4 |
��ش�
��1����ҺB�к��е���������__�������ӷ��ţ���
��2��������������������������X����__������ĸ����
A.NaClO B.H2O2 C.KMnO4
д������������Xʱ��������Ӧ�����ӷ���ʽ___��
��3�������Լ�����Ϊ�˵���pH���Լ��ٿ���ѡ��__���ѧʽ����
��֪����������Һ�е�����Ũ��С��1��10-5mol/Lʱ�������ʹ���ȫ����Fe(OH)3���ܶȻ�����Ksp=___��
��4�������ٵIJ��裺___��___�����ˡ�ϴ�ӡ����
��5������D����������Եõ�FeCl3������FeCl3��Һ��������˵������ȷ����__��
A.��FeCl3������Һ��μ����ˮ�У����������ȵõ����ɫҺ�壬��Һ���ܲ��������ЧӦ
B.��FeCl3��Һ�μӵ�����-KI��Һ�У���Һ����ɫ
C.��FeCl3��Һ�������ɲ����գ��õ�FeCl3����
D.��FeCl3��Һ�еμ�KSCN��Һ����Һ�г��ֺ�ɫ����