��Ŀ����
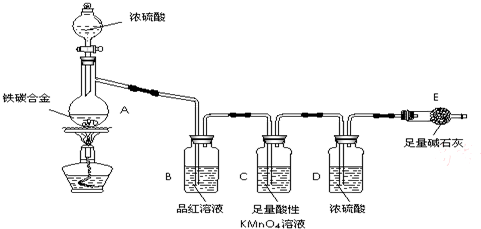
ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ�������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��ʵ��װ�ú�ʵ�鷽�����г�������ʡ�ԣ�����ݴ���ش���Ӧ���⡣
��̽��Ũ�����ijЩ����
��1����ͼʾ����װ�ã����װ�õ������ԣ�����E��������
��2����a g��̼�Ͻ���Ʒ����A�У��ټ���������Ũ���ᡣ����A������Ϊ____________��
δ��ȼ�ƾ���ǰ��A��B��������������ԭ���ǣ�____ ___��
��3����ȼ�ƾ���һ��ʱ���A��B�пɹ۲쵽��������
A�п�ʼ������Ӧ�Ļ�ѧ����ʽΪ��2Fe +6H2SO4 Fe2��SO4��3 + 3SO2�� +6H2O
Fe2��SO4��3 + 3SO2�� +6H2O
��______________________________________ ��д��ѧ����ʽ����
B�е�������_________,�ɴ˿ɵõ�Ũ�������_______�ԣ�Cװ�õ����� ��
��4�����ŷ�Ӧ�Ľ��У�A�л����ܷ���ijЩ���ӷ�Ӧ��д����Ӧ�����ӷ���ʽ__ __��
��5����Ӧһ��ʱ���A���ݳ������������Ȼ�Ͽ죬�����¶Ƚϸߣ���Ӧ�����⣬�����ܵ�ԭ����_____________________________________��
��ⶨ������������
��6����A�в����ݳ�����ʱ��ֹͣ���ȣ�����E�����ء�E����b g��
��̼�Ͻ���������������Ϊ_____________________(д����ʽ)��Ϊʹʵ�����ݸ�Ϊ��ȷ������װ�м�ʯ�ҵĸ���ܺ����________________________________��
(2) ������ƿ Fe��Ũ�����жۻ���C��Ũ�����ڳ����²���Ӧ
��3��C + 2H2SO4 CO2 + 2SO2�� +2H2O ��ɫ ������ ��ȥSO2������SO2�ѳ���
CO2 + 2SO2�� +2H2O ��ɫ ������ ��ȥSO2������SO2�ѳ���
��4��Fe + 2H+ �� Fe2+ + H2�� Fe+2Fe3+ �� Fe2+
��5����̼�Ͻ��γ�ԭ��ؼӿ췴Ӧ����
��6��1-3b/11a ��һ��װ��������ʯ�ҵĸ����
����

 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
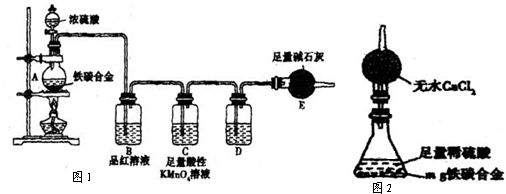
 ��5��������ҵ����ۣ�����������ͼװ�ã���������Լ�Ϊ��ˮ�Ȼ��ƣ���ƿ��Ϊmg��̿�Ͻ������ϡ���ᣮ������������ʵ�������ⶨijЩ���ݼ��ɣ�Ϊ�˿��ٲ�����������������������ʵ�������
��5��������ҵ����ۣ�����������ͼװ�ã���������Լ�Ϊ��ˮ�Ȼ��ƣ���ƿ��Ϊmg��̿�Ͻ������ϡ���ᣮ������������ʵ�������ⶨijЩ���ݼ��ɣ�Ϊ�˿��ٲ�����������������������ʵ�������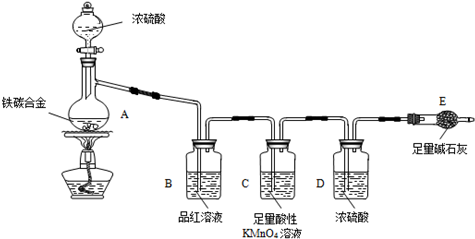
 ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ𣨿�������̼���ֵ��ʵĻ���������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��װ�ã��г�������ʡ�ԣ��ͷ�������ʵ�飮
ij��ѧ��ȤС��Ϊ�˲ⶨij��̼�Ͻ𣨿�������̼���ֵ��ʵĻ���������������������̽��Ũ�����ijЩ���ʣ��������ͼ��ʾ��װ�ã��г�������ʡ�ԣ��ͷ�������ʵ�飮