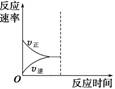
��Ŀ����
���Ṥҵβ���ж�������������0.05%�����������ʱ�辭����������ŷš�ijУ��ȤС�����ⶨ���Ṥҵβ���ж������������������·�����
����������ͼ��ʾ��ͼ������������B����ȷ����ͨ��β�����������β��ͨ��һ�������֪Ũ�ȵĵ�ˮ�вⶨ��������ĺ�����
��1��ϴ��ƿC�е���ĩ������һ���������D���������ʵ��ȷ�ȣ��������ǣ�
��
��2��ϴ��ƿC�е���Һ�����������Լ����������ٳ�һ�֣� ��
��3����ʵ��Ĺؼ����ڹر����������Ƶ�ʱ��,����Ӧ����ʲôʱ��رջ���A: ��
�ҷ�����ʵ�鲽������������ͼ��ʾ��
��4��д��������з�Ӧ�Ļ�ѧ����ʽ ��
��5������жϲ�����г����Ƿ���ȫ�ķ��� ��
��6��ͨ����β�����ΪVL���ѻ���ɱ�״����ʱ����β���ж��������������������
Ϊ ���ú���V��m�Ĵ���ʽ��ʾ����
�����������ҷ����в����ʡ�ԣ�ֱ�ӽ�β��ͨ�����Ba(OH)2��Һ�����ಽ���뷽������ͬ��
��7������Ϊ�������Ƿ������˵�����ɣ�
��
(15��)
��1��������������Һ�ĽӴ������������SO2���ˮ��ַ�Ӧ��2�֣�
��2�����Ը��������Һ�� ��2�֣�
��3����ϴ��ƿC����Һ��ɫ��ʧʱ��2�֣�
��4��SO2+H2O2==H2SO4��2�֣�
��5�����ú�,���ϲ���ҹ����Ba(OH)2 ,��δ���ְ�ɫ����,�������ȫ(2��)
��6��22.4m / (233V)��3�֣�
��7����������BaSO3����������ΪBaSO4 ��2�֣�
����:��1�����ö�����ݿ�����Ӧ��ĽӴ������ʹ���ʳ�ַ�Ӧ����2��SO2�����տ��úܶ����������Ը��������Һ����ˮ������������Һ��Ҫ�����ȣ���3��������Cװ����ɫ��ʧ�����رջ�������4���ҷ�����ԭ������H2O2��SO2����Ϊ���ᣬȻ����Ba(OH)2��Ӧ���ٸ��ݳ�����������SO2��������5��������ȫ�ˣ�����Һ����SO42-����Һ����Ba(OH)2�����ú�,���ϲ���Һ����Ba(OH)2,��δ���ְ�ɫ��������������ƣ��а�ɫ���� ,�������ȫ����6��n(SO2)=n(BaSO4)=m/233mol,V(SO2)=22.4m/233.��7�����ҷ����в����ʡ��,��SO2��Ba(OH)2��Ӧ���ɵ�BaSO3�ں���IJ����в��ֱ�����ΪBaSO4�����ֲ�֪BaSO3��BaSO4�ı�ֵ��������SO2������

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д���1����ҵ����Na2SO3��Һ������ҵβ���е�SO2���������ݱ�ʾ��Ӧ������
| n(SO32-) |
| n(HSO3-) |
|
91��9 | 1��1 | 9��91 | ||
| ������pH | 8.2 | 7.2 | 6.2 |
| n(SO32-) |
| n(HSO3-) |
��2����֪Ki1��H2SO3����Ki��HAc����Ki2��H2SO3����Ki2��H2CO3����ҪʹNaHSO3��Һ��c��Na+����c��HSO3-���ӽ�1��1��������Һ�м�������
a��H2SO3��Һ b��NaOH��Һ c�������� d��Na2CO3
��3��ʵ����ͨ�����µ��KHSO4��Һ�Ʊ����������K2S2O8��д������KHSO4�ĵ��뷽��ʽ��
��4��S2O82-��ǿ�����ԣ���ԭ����ΪSO42-�������̣�MnSO4��������أ�K2S2O8����������Һ�������Ӵ��¿ɷ�����Ӧ���õ��Ϻ�ɫ��Һ����д�˷�Ӧ�Ļ�ѧ����ʽ��
��5����֪��S2O32-�н�ǿ�Ļ�ԭ�ԣ�ʵ���ҿ���I-�ⶨK2S2O8��Ʒ�Ĵ��ȣ���Ӧ����ʽΪ��
S2O82-+2I-��2SO42-+I2 ���٣�
I2+2S2O32-��2I-+S4O62-���ڣ�
��S2O82-��S4O62-��I2������ǿ��˳��
��6��K2S2O8��ƫ����ϩ��CH2=CF2���ۺϵ���������ƫ����ϩ��CH3-CClF2������ȥHCl�Ƶã�����0.5molƫ����ϩ����Ҫ����54kJ���ȣ�д����Ӧ���Ȼ�ѧ����ʽ
��Ԫ�صĺ��������ڹ�ҵ����;�㷺�����������ա�
��ҵ����Na2SO3��Һ������ҵβ���е�SO2���±����ݱ�ʾ��Ӧ������ ��pH�仯�Ĺ�ϵ��
��pH�仯�Ĺ�ϵ��
|
|
91:9 |
1:1 |
9:91 |
|
������pH |
8.2 |
7.2 |
6.2 |
��1������ = 1ʱ����ҺpH= 7.2��ԭ��___________________������0.20 mol/L ��NaOH��Һ����Ӧǰ����Һ������䣩����SO2������Ӧ����Һ�����ԣ���
= 1ʱ����ҺpH= 7.2��ԭ��___________________������0.20 mol/L ��NaOH��Һ����Ӧǰ����Һ������䣩����SO2������Ӧ����Һ�����ԣ���
c (HSO3-) + 2c (SO32-) = _______ mol/L ��
��2����֪��Ki1(H2SO3)> Ki(HAc) > Ki2(H2SO3) > Ki2(H2CO3)��ҪʹNaHSO3��Һ��c(Na+):c(HSO3-)�ӽ�1:1��������Һ�м�������____________��
a��H2SO3��Һ b��NaOH��Һ c�������� d��Na2CO3
��3��ʵ����ͨ�����µ��KHSO4��Һ�Ʊ����������K2S2O8��д������KHSO4�ĵ��뷽��ʽ��__________________________________________��
��4��S2O82-��ǿ�����ԣ���ԭ����ΪSO42-�������̣�MnSO4��������أ�K2S2O8����������Һ�������Ӵ��¿ɷ�����Ӧ���õ��Ϻ�ɫ��Һ����д�˷�Ӧ�Ļ�ѧ����ʽ�� ��
��5����֪��S2O32-�н�ǿ�Ļ�ԭ�ԣ�ʵ���ҿ���I-�ⶨK2S2O8��Ʒ�Ĵ��ȣ���Ӧ����ʽΪ��
S2O82-��2I-��2SO42-��I2 ������ I2��2S2O32-��2I-��S4O62- ������
S2O82-��S4O62-��I2������ǿ��˳��__________________________��
��6��K2S2O8��ƫ����ϩ��CH2=CF2���ۺϵ���������ƫ����ϩ��CH3��CClF2������ȥHCl�Ƶã�����0.5 molƫ����ϩ����Ҫ����54 kJ���ȣ�д����Ӧ���Ȼ�ѧ����ʽ_______��


 Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O
Fe��OH��3+3H+ 2H++CaCO3=Ca2++CO2��+H2O Mn3O4+SO2��+2SO3��+3H2O
Mn3O4+SO2��+2SO3��+3H2O 2SO3(g)����H��0��
2SO3(g)����H��0��