��Ŀ����
[��ѧ--���ʽṹ������
��1��ԭ������ͬ���۵�����Ҳ��ͬ��������Ϊ�ȵ����壮�ȵ�����������ƵĻ�ѧ���������������ƣ�CO�ĽṹʽΪ______��
��2���Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸�����캽�ղ��ϣ�����Ϊ��δ������Ľ������� �ѵĻ�̬ԭ�ӵĵ����Ų�ʽΪ______��
��3��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж��С�TiӦ�ã�ƫ���ᱵΪ���Ӿ��壬�����Ľṹ��ͼ ��ʾ�����Ļ�ѧʽ��______��
��4�����к�Ti3+��������ѧʽΪTiCl3��H2O��6����1mol����������ˮ������������������Һ�������������Ȼ���Ϊl mol����֪����������λ��Ϊ6�������������λ����______��1mol�������������� �ᾧˮ�����ʵ���Ϊ______mol��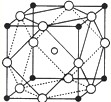
��1��ԭ������ͬ���۵�����Ҳ��ͬ��������Ϊ�ȵ����壮�ȵ�����������ƵĻ�ѧ���������������ƣ�CO�ĽṹʽΪ______��
��2���Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸�����캽�ղ��ϣ�����Ϊ��δ������Ľ������� �ѵĻ�̬ԭ�ӵĵ����Ų�ʽΪ______��
��3��ƫ���ᱵ��С�ͱ�ѹ������Ͳ���������ж��С�TiӦ�ã�ƫ���ᱵΪ���Ӿ��壬�����Ľṹ��ͼ ��ʾ�����Ļ�ѧʽ��______��
��4�����к�Ti3+��������ѧʽΪTiCl3��H2O��6����1mol����������ˮ������������������Һ�������������Ȼ���Ϊl mol����֪����������λ��Ϊ6�������������λ����______��1mol�������������� �ᾧˮ�����ʵ���Ϊ______mol��

��1��CO��N2Ϊ�ȵ����壬��֪N2�ĽṹʽΪN��N���ȵ�����������ƵĻ�ѧ����������CO�ĽṹʽΪC��O���ʴ�Ϊ��C��O��
��2��Ti��ԭ������Ϊ22�������������ԭ���ͺ��ع���Ti�ĵ����Ų�ʽΪ1s22s22p63s23p63d24s2��
�ʴ�Ϊ��1s22s22p63s23p63d24s2��
��3�����ݾ����Ľṹ�����þ�̯�����㣬�����к���һ��Ba2+��λ�ھ��������ģ���Ti4+λ�ڶ��㣬��ƽ������Ti4+�ĸ���Ϊ8��
=1��O2-λ���⣬ƽ��һ����������O2-�ĸ���Ϊ12��
=3�����Ի�ѧʽΪBaTiO3���ʴ�Ϊ��BaTiO3��
��4����1molTiCl3��H2O��6����ˮ������������������Һ�������������Ȼ���Ϊlmol��˵�����Cl-Ϊ1mol������2molCl-��Ti3+�γ���λ������֪����������λ��Ϊ6������4molH2O��Ti3+�γ���λ����1mol���������������ᾧˮ�����ʵ���Ϊ2mol��
�ʴ�Ϊ��Cl-��H2O��2��
��2��Ti��ԭ������Ϊ22�������������ԭ���ͺ��ع���Ti�ĵ����Ų�ʽΪ1s22s22p63s23p63d24s2��
�ʴ�Ϊ��1s22s22p63s23p63d24s2��
��3�����ݾ����Ľṹ�����þ�̯�����㣬�����к���һ��Ba2+��λ�ھ��������ģ���Ti4+λ�ڶ��㣬��ƽ������Ti4+�ĸ���Ϊ8��
| 1 |
| 8 |
| 1 |
| 4 |
��4����1molTiCl3��H2O��6����ˮ������������������Һ�������������Ȼ���Ϊlmol��˵�����Cl-Ϊ1mol������2molCl-��Ti3+�γ���λ������֪����������λ��Ϊ6������4molH2O��Ti3+�γ���λ����1mol���������������ᾧˮ�����ʵ���Ϊ2mol��
�ʴ�Ϊ��Cl-��H2O��2��

��ϰ��ϵ�д�
�����Ŀ

 ����ʾ�þ����е�һ��ԭ�ӣ����ڸ�������Ķ������á�
����ʾ�þ����е�һ��ԭ�ӣ����ڸ�������Ķ������á� ����ʾ����֮���ڵ�ԭ�ӣ�
����ʾ����֮���ڵ�ԭ�ӣ�

 ��ƽ��ṹ������
��ƽ��ṹ������ ��2013?�����ģ��[��ѧ-���ʽṹ������]
��2013?�����ģ��[��ѧ-���ʽṹ������]