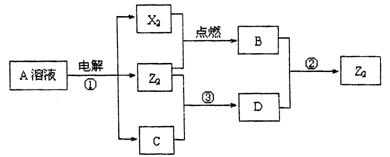
��Ŀ����
����14�֣���.�ñ�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�÷�̪��ָʾ�������в����лᵼ��ʵ����ƫ�͵��� (����� )
�ټ�ʽ�ζ���������ˮϴ����û���ñ�Һ��ϴ
������ʽ�ζ��ܼӴ���Һʱ����������ˮϴ����ĵζ���δ�ô���Һ��ϴ
����ƿ������ˮϴ����û���ô���Һ��ϴ
�ܵζ�ǰ�ζ��ܼ�������ݣ��ζ���������ʧ
���յ����ʱ���ӣ���������������ȷ
��.����Fe(OH)2���ױ�����������ʵ���Һ�������������Һ���ռӦ�Ƶð�ɫ������Fe(OH)2����������ͼ��ʾʵ��װ������Ƶô�����Fe(OH)2�������������Ϸֱ�Ϊʯī������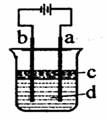
��1��a�缫����Ϊ ���õ缫��Ӧʽ
��2������ɫ�����ڵ缫�����ɣ�����Һd�� ������ɫ����������֮�����Һ�����ɣ�����Һd��________������ĸ���ţ�
A����ˮ��B��NaCl��Һ��C��NaOH��Һ��D��CuCl2��Һ
��3��Һ��cΪ������������
��4����������֮�����Һ���ܶ�ʱ���ڿ�����ɫ���������Բ�ȡ�Ĵ�ʩ��________________��
A������ϡ���������Һ������B���ʵ������Դ��ѹ ����C���ʵ����͵��Һ�¶�
��.�ڢ�
��.��1��Fe Fe ��2e�� = Fe2+
��2��C��B
��3��������������ֹ��ɫ����������
��4��B
����