��Ŀ����
����Ŀ����ͳ���Ʊ��ϳɰ�ԭ����H2�ķ�����ˮú��������֪��
��C(s)+H2O(g)=CO(g)+H2(g) ��H1=��131.3kJ��mol��1
��C(s)+2H2O(g)=CO2(g)+2H2(g) ��H2=��90.2kJ��mol��1
��CO(g)+H2O(g)=CO2(g)+H2��g�� ��H3
��1����Ӧ�١�H1��0����һ�������£����Է����е�ԭ����_______________����H3=__________kJ��mol��1��
��2����֪800��ʱ��CO(g)+H2O(g)![]() CO2(g)+H2��g�� K=1.0���ڴ��¶�����һ2L�ĺ��º����ܱ������г���2molH2O(g)��2molCO,2min��ﵽƽ�⣬��ƽ��ʱCO2�����ʵ���Ũ��Ϊ_______________���ӷ�Ӧ��ʼ���ﵽƽ��ʱH2O��ƽ����Ӧ����Ϊ_______________��
CO2(g)+H2��g�� K=1.0���ڴ��¶�����һ2L�ĺ��º����ܱ������г���2molH2O(g)��2molCO,2min��ﵽƽ�⣬��ƽ��ʱCO2�����ʵ���Ũ��Ϊ_______________���ӷ�Ӧ��ʼ���ﵽƽ��ʱH2O��ƽ����Ӧ����Ϊ_______________��
��3��900��ʱ����Ӧ��CO(g)+H2O(g) ![]() CO2(g)+H2(g)��ƽ�ⳣ��K__________1.0���������������������
CO2(g)+H2(g)��ƽ�ⳣ��K__________1.0���������������������
���𰸡� ��S��0 �������� ����ϵ���Ҷ����� -41.1 0.5 mol��L��1 0.25 mol��L��1��min��1 ����λд�������֣� ��
��������(1)�Է����е���������H-T��S��0����Ӧ��C(s)+H20(g)=CO(g)+H2(g)��H=+131.3kJ/mol�����ȷ�Ӧ����H��0����S��0�����Է�Ӧ�Է������Ǹ����½��еķ�Ӧ�����ݸ�˹���ɣ���-���õ�������H3=(��90.2kJ��mol��1)-(��131.3kJ��mol��1)=-41.1KJ/mol��
(2)���ݻ�ѧƽ������ʽ��ʽ���跴Ӧ�ﵽƽ������ˮ�����ʵ���Ϊx
CO(g)+H2O(g)C02(g)+H2(g)
��ʼ��(mol) 22 0 0
�仯��(mol) x x xx
ƽ����(mol) 2-x2-x x x
K=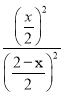 =1������õ�x=1��ƽ��ʱCO2�����ʵ���Ũ��Ϊ
=1������õ�x=1��ƽ��ʱCO2�����ʵ���Ũ��Ϊ![]() =0.5mol/L���ӷ�Ӧ��ʼ���ﵽƽ��ʱH2O��ƽ����Ӧ����Ϊ
=0.5mol/L���ӷ�Ӧ��ʼ���ﵽƽ��ʱH2O��ƽ����Ӧ����Ϊ![]() =0.25 mol��L��1��min��1 ��
=0.25 mol��L��1��min��1 ��
(3)900��ʱ��������ɷ�Ӧ��֪��Ӧ�Ƿ��ȷ�Ӧ������ƽ��������У���ӦCO(g)+H2O(g)C02(g)+H2(g)��ƽ�ⳣ����С��K��1.0��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д�