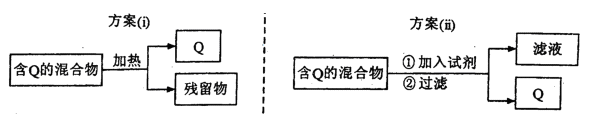
��Ŀ����
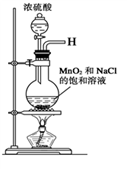
ijУ̽��ѧϰС��ͬѧ�ú�����������(��ҪΪ������ɳ��CaCl2��MgCl2��Na2SO4��)�Ĵ�����ȡ����ѧ��������NaCl��ʵ��ǰ������������·���(��ͼ)��
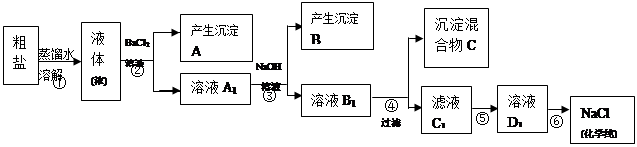
(1)��д�������ڢܡ��ݲ������Լ����Ƽ��ڢ��������ƣ��� ���� ���� ��
(2) ���������C�Ļ�ѧ�ɷ���(�����ֺͻ�ѧʽ��ʾ)�� ��
(3)д���ڢݲ������п��ܷ�����Ӧ�����ӷ���ʽ��
��
(4)��������������ڢݲ�ʵ���Ƿ�ﵽ��Ŀ�ģ�
��
(5)����Ϊ���������Щ���������Ӱ��ʵ������ ��
(6)��ͬѧ��Ϊ����ʵ����Ʋ�����Լ����������һ�����룺
��

(1)��д�������ڢܡ��ݲ������Լ����Ƽ��ڢ��������ƣ��� ���� ���� ��
(2) ���������C�Ļ�ѧ�ɷ���(�����ֺͻ�ѧʽ��ʾ)�� ��
(3)д���ڢݲ������п��ܷ�����Ӧ�����ӷ���ʽ��
��
(4)��������������ڢݲ�ʵ���Ƿ�ﵽ��Ŀ�ģ�
��
(5)����Ϊ���������Щ���������Ӱ��ʵ������ ��
(6)��ͬѧ��Ϊ����ʵ����Ʋ�����Լ����������һ�����룺
��
�Ţ�̼������Һ ������ �������������ᾧҲ�ɣ�����1�֣�
�� ��ɳ��BaSO4��BaCO3��CaCO3��Mg(OH)2��2�֣�
�ǵڢݲ���H+ + OH- = H2O ��1�֣� CO32- + 2H+ = H2O + CO2����1�֣�
�� �ò�����պȡ��Һ����pH��ֽ�в�������죬˵����Һ�����ԣ���OH-��CO32-���ڣ���������˵����Һ�Գʼ��ԣ���OH-��CO32-���ڣ�������μ������������ԡ���2�֣�
�ɢں͢ۻ�ۺ͢ܣ�2�֣�
�� ��Ba (OH)2����BaCl2��NaOH��ʹ�ڢ۲��ϲ�Ϊһ����1�֣�
�����������1����������ͼ�Ѹ������Լ������ã�BaCl2��Һ��ȥNa2SO4��NaOH��Һ��ȥMgCl2�����Ԣܵ�����Ϊ��ȥCaCl2����ѡ�Լ�ΪNa2CO3��Һ���ݵ������dz�ȥ������NaOH��Na2CO3��Ӧѡ�����ᣬ�������Ǵ�NaCl��Һ�еõ���ѧ��NaCl��Ϊ����������
��2��BaCl2��NaOH��Na2CO3��Ӧ��õ��ij������У���ɳ��BaSO4��BaCO3��CaCO3��Mg(OH)2��
��3���ڢݲ�����HCl����NaOH��Na2CO3��Ӧ���������ӷ���ʽΪ��H+ + OH- = H2O�� CO32- + 2H+ = H2O + CO2����
��4��HCl����ʱ�ڢݲ��ﵽ��Ŀ�ģ�����pH��ֽ���飬����ֽ�仯��֤��HCl������OH?��CO32?�����ڣ���������˵������OH?��CO32?��������μ������������ԡ�
��5��NaOH��Ӱ���������ʵij�ȥ������NaOH�ļ���˳�������ǰ��Ҳ���������������ں͢ۻ�ۺܿ͢ɵ�����
��6����Ba (OH)2����BaCl2��NaOH����ͬʱ��ȥNa2SO4��MgCl2��ʹ�ڢ۲��ϲ�Ϊһ����

��ϰ��ϵ�д�
 ����ȫ���ִʾ��ƪ��ϵ�д�
����ȫ���ִʾ��ƪ��ϵ�д� �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д�
�����Ŀ