��Ŀ����
����ʵ�������ʵ��Ŀ�Ļ����һ�µ��ǣ�������
| ѡ�� | ʵ����� | ʵ��Ŀ�Ļ���� |
| A | ��ij��Һ���ȵ����ϡ�����ữ���ٵμ�BaCl2��Һ���а�ɫ�������� | ����Һ��һ������Ag+ |
| B | ���������ͨ������Na2CO3��Һ | ��ȥCO2�л���HCl |
| C | ����SnCl2��Һʱ���Ƚ�SnCl2��������ϡ���ᣬ��������ˮϡ�ͣ�����ʱ���Լ�ƿ�м������������� | ����Sn2+ˮ�⣬����ֹSn2+������ΪSn4+ |
| D | ����������ˮ�У������ã���ˮ����ɫ | ��ͱ������ӳɷ�Ӧ |
������A���������ữ����Һ�д�����������ӻ�����������Ӿ�������ͬ������
B��������̼��HCl����̼������Һ��Ӧ��
C���������ƽ������ӵ�ˮ�⣬�����������������л�ԭ�ԣ���ֹSn2+��������
D���岻������ˮ�������ڱ���
B��������̼��HCl����̼������Һ��Ӧ��
C���������ƽ������ӵ�ˮ�⣬�����������������л�ԭ�ԣ���ֹSn2+��������
D���岻������ˮ�������ڱ���
����⣺A���������ữ����Һ�д�����������ӻ�����������ӣ��ٵμ�BaCl2��Һ�����а�ɫ�������ɣ�����ȷ������Ag+��Ӧ�ȼ������ữ����A����
B��������̼��HCl����̼������Һ��Ӧ����ѡ̼��������Һ����ȥCO2�л���HCl����B����
C�����������ˮ�⣬���ױ���������������ƽ������ӵ�ˮ�⣬�����������������л�ԭ�ԣ���ֹSn2+����������C��ȷ��
D���岻������ˮ�������ڱ�����������ˮ�У������ã���ˮ����ɫ��������ȡ��Ϊ�����仯����D����
��ѡC��
B��������̼��HCl����̼������Һ��Ӧ����ѡ̼��������Һ����ȥCO2�л���HCl����B����
C�����������ˮ�⣬���ױ���������������ƽ������ӵ�ˮ�⣬�����������������л�ԭ�ԣ���ֹSn2+����������C��ȷ��
D���岻������ˮ�������ڱ�����������ˮ�У������ã���ˮ����ɫ��������ȡ��Ϊ�����仯����D����
��ѡC��
���������⿼��ʵ�鷽�������ۣ��漰�����ӵļ��顢��ȡ�����ʵij��ӡ�����ˮ�⡢�Լ��Ĵ�ŵ�֪ʶ�㣬���ڳ�������ƴ���⣬�����϶࣬ѧ��Ӧע��˼ά�ļ�ʱת�������AΪ�����״��㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 ijͬѧ��������װ��ʵ��ͭ��Ũ���ᡢϡ���ᷴӦ��������ͼ��ʾ��
ijͬѧ��������װ��ʵ��ͭ��Ũ���ᡢϡ���ᷴӦ��������ͼ��ʾ�� ��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺
��˵�����¾��ϴ��ر��㡱����ԭ���Ǿ��ڴ������������������ζ��������������ʵ����������Ҳ��������ͼ��ʾ��װ����ģ��ù��̣���ش��������⣺



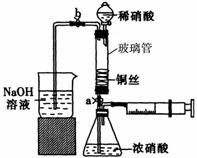 ��ϴ�Ӻ��ͭ˿���������϶������״��
��ϴ�Ӻ��ͭ˿���������϶������״��