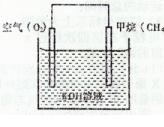
题目内容
已知热化学方程式:2H2(g)+O2(g)===2H2O(g) ΔH1=-483.6 kJ/mol,则对于热化学方程式:2H2O(l)===2H2(g)+O2(g) ΔH2=b,下列说法正确的是( )
| A.热化学方程式中化学计量数表示分子个数 |
| B.该反应的ΔH2=+483.6 kJ/mol |
| C.|ΔH2|<|ΔH1| |
| D.|ΔH2|>|ΔH1| |
D
A错,热化学方程式中化学计量数表示物质的量;B错,2H2O(g)===2H2(g)+O2(g) 的ΔH=+483.6 kJ/mol;C错,D正确,液态水变成气态水要吸收能量,所以|ΔH2|>|ΔH1|

练习册系列答案
相关题目


 。
。 CH3OH(g)△H<0,其化学平衡常数K和温度T的关系如下表所示:
CH3OH(g)△H<0,其化学平衡常数K和温度T的关系如下表所示: 2NH3 ( g ) △H =" -92.0" kJ/mol,将此温度下的1 mol N2和3 mol H2放在一密闭容器中,在催化剂存在时进行反应,测得反应放出的热量为( )
2NH3 ( g ) △H =" -92.0" kJ/mol,将此温度下的1 mol N2和3 mol H2放在一密闭容器中,在催化剂存在时进行反应,测得反应放出的热量为( ) 
 ClO(g)+3H2(g)△H>0。
ClO(g)+3H2(g)△H>0。