��Ŀ����
����Һ�У���ӦA+2B![]() C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ
C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ![]() ��
��![]() ��
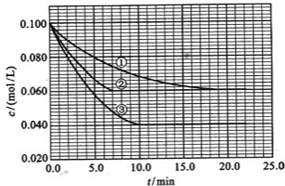
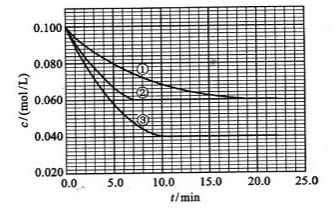
��![]() ����Ӧ��A��Ũ����ʱ��ı仯����ͼ��ʾ��
����Ӧ��A��Ũ����ʱ��ı仯����ͼ��ʾ��
��ش��������⣺
��1����ٱȽϣ��ں͢۷ֱ���ı�һ�ַ�Ӧ���������ı���������жϵ������ǣ�
��_______________��
��_______________��
��2��ʵ���ƽ��ʱB��ת����Ϊ_________��ʵ���ƽ��ʱC��Ũ��Ϊ____________��
��3���÷�Ӧ��![]() _________0���ж���������__________________________________��
_________0�����������__________________________________��
��4���÷�Ӧ���е�4.0minʱ��ƽ����Ӧ�ٶ��ʣ�
ʵ��ڣ�![]() =__________________________________��
=__________________________________��
ʵ��ۣ�![]() =__________________________________��
=__________________________________��
��1���ڼӴ������ﵽƽ���ʱ�����̣�ƽ��ʱA��Ũ��δ��
���¶����ߣ��ﵽƽ���ʱ�����̣�ƽ��ʱA��Ũ�ȼ�С
��2��40%����0.4����0.06mol/L����3�����������¶����������ƶ����ʸ÷�Ӧ�����ȷ�Ӧ
��4��0.014mol(L��min)-1��0.008mol(L��min)-1
����:
��1����ʹ���ˣ��������������ɣ���Ϊ��ͼ��ɿ������������յ�ƽ��Ũ����ͬ�������յ�ƽ��״̬��ͬ�����ڱȢ�����Ҫ��ʱ��̣���Ȼ��Ӧ���ʼӿ��ˣ�����Ӱ�췴Ӧ���ʺ�Ӱ��ƽ������ؿ�֪�Ǽ��루�����������������¶ȣ����ɣ���Ϊ�÷�Ӧ������Һ�н��еķ�Ӧ�����Բ������Ǹı�ѹǿ�������ʵĸı䣬�����ڸ�������ʼŨ����ͬ���ʲ������Ǹı�Ũ��Ӱ�췴Ӧ���ʣ������ڢۺ͢���ȴ�ƽ������ʱ��̣�ƽ��ʱŨ�ȸ�С���ʲ������Ǹ��ô�������ֻ���������¶���Ӱ�췴Ӧ���ʵ�
��2����������ҺΪ1L������д�ƽ��ʱAת����0.04mol���ɷ�Ӧ��������֪Bת����0.08mol������Bת����Ϊ![]() ��ͬ���ڢ���Aת����0.06mol��������CΪ0.06mol,������䣬��ƽ��ʱC(c)=0.06mol/L
��ͬ���ڢ���Aת����0.06mol��������CΪ0.06mol,������䣬��ƽ��ʱC(c)=0.06mol/L
(3) ![]() ��0�����ɣ��ɢۺ͢ٽ��жԱȿ�֪�����¶Ⱥ�A��ƽ��Ũ�ȼ�С����A��ת�������ߣ�ƽ�����������ƶ����������������ȵķ����ƶ�����������Ӧ�����ȷ�Ӧ��
��0�����ɣ��ɢۺ͢ٽ��жԱȿ�֪�����¶Ⱥ�A��ƽ��Ũ�ȼ�С����A��ת�������ߣ�ƽ�����������ƶ����������������ȵķ����ƶ�����������Ӧ�����ȷ�Ӧ��![]() ��0
��0
��4����ͼ�϶��������е�4.0minʱ��ʵ��ڵ�A��Ũ��Ϊ��0.072mol/L,���C(A)=0.10-0.072=0.028mol/L��![]() ,��
,��![]() =2
=2![]() =0.014mol(L��min)-1�����е�4.0miʵ��۵�A��Ũ��Ϊ��0.064mol/L����C(A,) =0.10-0.064=0.036mol/L��
=0.014mol(L��min)-1�����е�4.0miʵ��۵�A��Ũ��Ϊ��0.064mol/L����C(A,) =0.10-0.064=0.036mol/L��![]() ,��
,��![]() =
=![]() =0.0089mol(L��min)-1
=0.0089mol(L��min)-1

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д� ��2010?����������Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L �� c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ��
��2010?����������Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊc��A��=0.100mol/L��c��B��=0.200mol/L �� c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ�� ����Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ c��A��=1.0mol/L��c��B��=2.0mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ����ش��������⣺
����Һ�У���ӦA+2B?C�ֱ������ֲ�ͬʵ�������½��У����ǵ���ʼŨ�Ⱦ�Ϊ c��A��=1.0mol/L��c��B��=2.0mol/L��c��C��=0mol/L����Ӧ��A��Ũ����ʱ��ı仯��ͼ��ʾ����ش��������⣺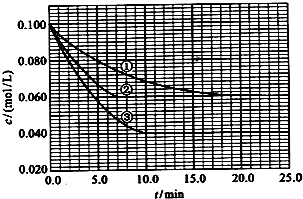 ��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ�
��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����ã���ҵ�Ͽ�����ú���������ˮú�����ϳɶ����ѣ� ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺