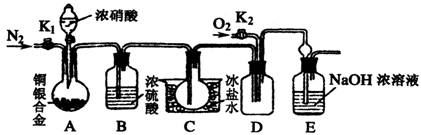
��Ŀ����
ij�о���ѧϰС����֤Ũ������ͭ���Ͻ�Ӧ�г�����NO2���������������NO���ɡ��������ϵ�֪��������NO2��N2O4��ϴ��ڣ��ڵ���0��ʱ����ֻ����ɫ��N2O4Һ�������ڡ�Ϊ�ˣ������������ͼ��ʾ����֤ʵ��װ��(ͼ����ȥ�˲���Ҫ��װ��)��
��ش��������⣺
(1)д��Aƿ��ͭ��������ܷ�����Ӧ�Ļ�ѧ����ʽ��______________________��
(2)ʵ�鿪ʼǰҪ�ȴ�A���ֵĻ���K1������ͨһ��ʱ��ĵ����ٹر�K1����
������Ŀ����______________________________________________��װ����Bƿ��������____________________________________________________________________��
(3)A�еķ�Ӧֹͣ��D�еĻ���K2����ͨ������������Ӧȷ��NO��������D��Ӧ���ֵ�������__________________��ʵ�鷢�֣�ͨ�������¶ȵĸߵͶ�ʵ�������нϴ�Ӱ�죬Ϊ���ڹ۲�Ӧͨ��___________(��䡱���ȡ�)��������
(4)��֪�Ͻ������Ϊa���ҺϽ���ȫ�ܽ⡣������ⶨͭ������������ֻ������Aװ������Ӧ�����Һ������ʵ������Ϳ��ԴﵽĿ�ġ������ʵ����̣�_____________________��
(1)Cu+4HNO3(Ũ)====Cu(NO3)2+2NO2![]() +2H2O
+2H2O
3Cu+8HNO3(ϡ)====3Ca(NO3)2+2NO![]() +4H2O
+4H2O
(2)�ų�ϵͳ�ڵĿ�������ֹһ�����������ɶ������� ����ˮ��������ֹˮ������C�������������������Ӧ����һ������
(3)���ֺ���ɫ���� ��
(4)��A�м�������ĺ�Cl-����Һ�����ˡ�ϴ�ӡ����ﲢ��ȡ����������(�ĸ����ֵ�ؼ��ʢٹ�������Cl-���۹��ˡ�ϴ�ӣ��ܸ������)

 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�